Development of brain structural connectivity between ages 12 and 30: a 4-Tesla diffusion imaging study in 439 adolescents and adults
- PMID: 22982357
- PMCID: PMC3603574
- DOI: 10.1016/j.neuroimage.2012.09.004
Development of brain structural connectivity between ages 12 and 30: a 4-Tesla diffusion imaging study in 439 adolescents and adults
Abstract
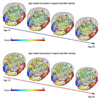
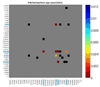
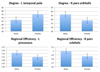
Understanding how the brain matures in healthy individuals is critical for evaluating deviations from normal development in psychiatric and neurodevelopmental disorders. The brain's anatomical networks are profoundly re-modeled between childhood and adulthood, and diffusion tractography offers unprecedented power to reconstruct these networks and neural pathways in vivo. Here we tracked changes in structural connectivity and network efficiency in 439 right-handed individuals aged 12 to 30 (211 female/126 male adults, mean age=23.6, SD=2.19; 31 female/24 male 12 year olds, mean age=12.3, SD=0.18; and 25 female/22 male 16 year olds, mean age=16.2, SD=0.37). All participants were scanned with high angular resolution diffusion imaging (HARDI) at 4 T. After we performed whole brain tractography, 70 cortical gyral-based regions of interest were extracted from each participant's co-registered anatomical scans. The proportion of fiber connections between all pairs of cortical regions, or nodes, was found to create symmetric fiber density matrices, reflecting the structural brain network. From those 70 × 70 matrices we computed graph theory metrics characterizing structural connectivity. Several key global and nodal metrics changed across development, showing increased network integration, with some connections pruned and others strengthened. The increases and decreases in fiber density, however, were not distributed proportionally across the brain. The frontal cortex had a disproportionate number of decreases in fiber density while the temporal cortex had a disproportionate number of increases in fiber density. This large-scale analysis of the developing structural connectome offers a foundation to develop statistical criteria for aberrant brain connectivity as the human brain matures.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




References
-
- Annett M. A classification of hand preference by association analysis. British Journal of Psychology. 1970;61(3):303–321. - PubMed
-
- Bartzokis G. Quadratic trajectories of brain myelin content: unifying construct for neuropsychiatric disorders. Neurobiology of Aging. 2004;25:49–62.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

