Promoter clearance by RNA polymerase II
- PMID: 22982364
- PMCID: PMC3529798
- DOI: 10.1016/j.bbagrm.2012.08.010
Promoter clearance by RNA polymerase II
Abstract
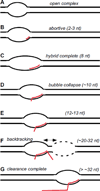
Many changes must occur to the RNA polymerase II (pol II) transcription complex as it makes the transition from initiation into transcript elongation. During this intermediate phase of transcription, contact with initiation factors is lost and stable association with the nascent transcript is established. These changes collectively comprise promoter clearance. Once the transcript elongation complex has reached a point where its properties are indistinguishable from those of complexes with much longer transcripts, promoter clearance is complete. The clearance process for pol II consists of a number of steps and it extends for a surprisingly long distance downstream of transcription start. This article is part of a Special Issue entitled: RNA polymerase II Transcript Elongation.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures
References
-
- Orphanides G, Lagrange T, Reinberg D. The general transcription factors of RNA polymerase II. Genes Dev. 1996;10:2657–2683. - PubMed
-
- Roeder RG. The role of general initiation factors in transcription by RNA polymerase II. Trends Biochem. Sci. 1996;21:327–335. - PubMed
-
- Kostrewa D, Zeller ME, Armache KJ, Seizl M, Leike K, Thomm M, Cramer P. RNA polymerase II-TFIIB structure and mechanism of transcription initiation. Nature. 2009;462:323–330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

