Ca(V)1.1: The atypical prototypical voltage-gated Ca²⁺ channel
- PMID: 22982493
- PMCID: PMC3615030
- DOI: 10.1016/j.bbamem.2012.09.007
Ca(V)1.1: The atypical prototypical voltage-gated Ca²⁺ channel
Abstract
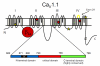
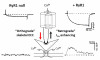
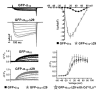
Ca(V)1.1 is the prototype for the other nine known Ca(V) channel isoforms, yet it has functional properties that make it truly atypical of this group. Specifically, Ca(V)1.1 is expressed solely in skeletal muscle where it serves multiple purposes; it is the voltage sensor for excitation-contraction coupling and it is an L-type Ca²⁺ channel which contributes to a form of activity-dependent Ca²⁺ entry that has been termed Excitation-coupled Ca²⁺ entry. The ability of Ca(V)1.1 to serve as voltage-sensor for excitation-contraction coupling appears to be unique among Ca(V) channels, whereas the physiological role of its more conventional function as a Ca²⁺ channel has been a matter of uncertainty for nearly 50 years. In this chapter, we discuss how Ca(V)1.1 supports excitation-contraction coupling, the possible relevance of Ca²⁺ entry through Ca(V)1.1 and how alterations of Ca(V)1.1 function can have pathophysiological consequences. This article is part of a Special Issue entitled: Calcium channels.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures

References
-
- Tanabe T, Takeshima H, Mikami A, Flockerzi V, Takahashi H, Kangawa K, Kojima M, Matsuo H, Hirose T, Numa S S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987;328:313–318. - PubMed
-
- Beam KG, Knudson CM, Powell JA. A lethal mutation in mice eliminates the slow calcium current in skeletal muscle cells. Nature. 1986;320:168–170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

