Thalamic glutamatergic afferents into the rat basolateral amygdala exhibit increased presynaptic glutamate function following withdrawal from chronic intermittent ethanol
- PMID: 22982568
- PMCID: PMC3521082
- DOI: 10.1016/j.neuropharm.2012.09.004
Thalamic glutamatergic afferents into the rat basolateral amygdala exhibit increased presynaptic glutamate function following withdrawal from chronic intermittent ethanol
Abstract
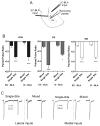
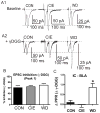
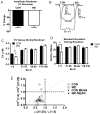
Amygdala glutamatergic neurotransmission regulates withdrawal induced anxiety-like behaviors following chronic ethanol exposure. The lateral/basolateral amygdala receives multiple glutamatergic projections that contribute to overall amygdala function. Our lab has previously shown that rat cortical (external capsule) afferents express postsynaptic alterations during chronic intermittent ethanol exposure and withdrawal. However, thalamic (internal capsule) afferents also provide crucial glutamatergic input during behavioral conditioning, and they have not been studied in the context of chronic drug exposure. We report here that these thalamic inputs express altered presynaptic function during withdrawal from chronic ethanol exposure. This is characterized by enhanced release probability, as exemplified by altered paired-pulse ratios and decreased failure rates of unitary events, and increased concentrations of synaptic glutamate. Quantal analysis further implicates a withdrawal-dependent enhancement of the readily releasable pool of vesicles as a probable mechanism. These functional alterations are accompanied by increased expression of vesicle associated protein markers. These data demonstrate that chronic ethanol modulation of glutamate neurotransmission in the rat lateral/basolateral amygdala is afferent-specific. Further, presynaptic regulation of lateral/basolateral amygdala thalamic inputs by chronic ethanol may be a novel neurobiological mechanism contributing to the increased anxiety-like behaviors that characterize withdrawal.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
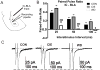


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

