Phase I study of nelfinavir in liposarcoma
- PMID: 22983015
- PMCID: PMC3904496
- DOI: 10.1007/s00280-012-1961-4
Phase I study of nelfinavir in liposarcoma
Abstract
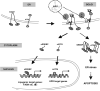
Purpose: HIV protease inhibitors are associated with HIV protease inhibitor-related lipodystrophy syndrome. We hypothesized that liposarcomas would be similarly susceptible to the apoptotic effects of an HIV protease inhibitor, nelfinavir.
Methods: We conducted a phase I trial of nelfinavir for liposarcomas. There was no limit to prior chemotherapy. The starting dose was 1,250 mg twice daily (Level 1). Doses were escalated in cohorts of three to a maximally evaluated dose of 4,250 mg (Level 5). One cycle was 28 days. Steady-state pharmacokinetics (PKs) for nelfinavir and its primary active metabolite, M8, were determined at Levels 4 (3,000 mg) and 5.
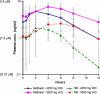
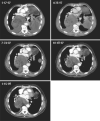
Results: Twenty subjects (13 males) were enrolled. Median (range) age was 64 years (37-81). One subject at Level 1 experienced reversible, grade 3 pancreatitis after 1 week and was replaced. No other dose-limiting toxicities were observed. Median (range) number of cycles was 3 (0.6-13.5). Overall best responses observed were 1 partial response, 1 minor response, 4 stable disease, and 13 progressive disease. Mean peak plasma levels and AUCs for nelfinavir were higher at Level 4 (7.3 mg/L; 60.9 mg/L × h) than 5 (6.3 mg/L; 37.7 mg/L × h). The mean ratio of M8:nelfinavir AUCs for both levels was ~1:3.
Conclusions: PKs demonstrate auto-induction of nelfinavir clearance at the doses studied, although the mechanism remains unclear. Peak plasma concentrations were within range where anticancer activity was demonstrated in vitro. M8 metabolite is present at ~1/3 the level of nelfinavir and may also contribute to the anticancer activity observed.
Figures
References
-
- Fletcher CDM, Rydholm A, Singer S, Sudaram M, Coindre JM. Soft tissue tumours: epidemiology, clinical features, histopathological typing and grading. In: Fletcher CDM, Unni KK, Mertens F, editors. Pathology & genetics: tumours of soft tissue and bone. IARC Press; Lyon: 2002. pp. 9–19.
-
- Flexner C. HIV-protease inhibitors. N Engl J Med. 1998;338:1281–1292. - PubMed
-
- Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidemia, and diabetes mellitus. Lancet. 1999;353:2093–2099. - PubMed
-
- Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naïve patients. JAMA. 2004;29(2):191–201. - PubMed
-
- Brinkman K, Smeitink JA, Romjin JA, Reiss P. Mitochondrial toxicity induced by nucleoside-analogue reverse-transcriptase inhibitors is a key factor in the pathogenesis of antiretroviral-therapy-related lipodystrophy. Lancet. 1999;354:1112–1115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

