Sirt1: def-eating senescence?
- PMID: 22983125
- PMCID: PMC3524209
- DOI: 10.4161/cc.22074
Sirt1: def-eating senescence?
Abstract
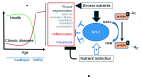
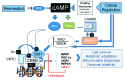
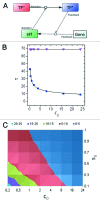
Sirt1, the closest mammalian homolog of the Sir2 yeast longevity protein, has been extensively investigated in the last few years as an avenue to understand the connection linking nutrients and energy metabolism with aging and related diseases. From this research effort the picture has emerged of an enzyme at the hub of a complex array of molecular interactions whereby nutrient-triggered signals are translated into several levels of adaptive cell responses, the failure of which underlies diseases as diverse as diabetes, neurodegeneration and cancer. Sirt1 thus connects moderate calorie intake to "healthspan," and a decline of Sirt-centered protective circuits over time may explain the "catastrophic" nature of aging.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
