Cancer-testis antigen expression and immunogenicity in AL amyloidosis
- PMID: 22983433
- PMCID: PMC3461704
- DOI: 10.1038/bcj.2012.32
Cancer-testis antigen expression and immunogenicity in AL amyloidosis
Abstract
Light-chain amyloidosis (AL) is a plasma cell dyscrasia closely related to multiple myeloma. In multiple myeloma, the cancer-testis antigens (CTAs) CT7 (MAGE-C1), CT10 (MAGE-C2) and MAGE-A CTAs are expressed in up to 80% of cases. In this study, we investigated the expression and immunogenicity of several CTAs in patients with AL amyloidosis in a total of 38 bone marrow specimens by employing standard immunohistochemistry techniques on paraffin-embedded archival tissues. Plasma samples from 35 patients (27 with matched bone marrow samples) were also analyzed by ELISA for sero reactivity to a group of full-length CTA proteins. CT7 was present in 25/38 (66%) while CT10 was demonstrated in 3/38 and GAGE in 1/38 AL amyloid cases. The expression pattern was mostly focal. There were no significant differences with regard to organ involvement, response to treatment, or prognosis in CTA positive compared to negative cases. None of the specimens showed spontaneous humoral immunity to CT7, but sero reactivity was observed in individual patients to other CTAs. This study identifies CT7 as the prevalent CTA in plasma cells of patients with AL amyloidosis. Further analyses determining the biology of CTAs in AL amyloidosis and their value as potential targets for immunotherapy are warranted.
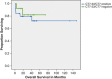
Figures

Similar articles
-
MAGE-C1/CT7 and MAGE-C2/CT10 are frequently expressed in multiple myeloma and can be explored in combined immunotherapy for this malignancy.Cancer Immunol Immunother. 2013 Jan;62(1):191-5. doi: 10.1007/s00262-012-1376-4. Epub 2012 Nov 22. Cancer Immunol Immunother. 2013. PMID: 23180015 Free PMC article.
-
An overview of cancer/testis antigens expression in classical Hodgkin's lymphoma (cHL) identifies MAGE-A family and MAGE-C1 as the most frequently expressed antigens in a set of Brazilian cHL patients.BMC Cancer. 2011 Sep 28;11(1):416. doi: 10.1186/1471-2407-11-416. BMC Cancer. 2011. PMID: 21951388 Free PMC article.
-
Expression and prognostic relevance of MAGE-C1/CT7 and MAGE-C2/CT10 in osteolytic lesions of patients with multiple myeloma.Exp Mol Pathol. 2010 Oct;89(2):175-81. doi: 10.1016/j.yexmp.2010.06.011. Epub 2010 Jul 14. Exp Mol Pathol. 2010. PMID: 20621094
-
Cancer/Testis Antigen MAGE-C1/CT7: new target for multiple myeloma therapy.Clin Dev Immunol. 2012;2012:257695. doi: 10.1155/2012/257695. Epub 2012 Mar 11. Clin Dev Immunol. 2012. PMID: 22481966 Free PMC article. Review.
-
Expression of cancer/testis antigens in cutaneous melanoma: a systematic review.Melanoma Res. 2019 Aug;29(4):349-357. doi: 10.1097/CMR.0000000000000569. Melanoma Res. 2019. PMID: 30615012
Cited by
-
Reverse Vaccinology Approach in Constructing a Multi-Epitope Vaccine Against Cancer-Testis Antigens Expressed in Non-Small Cell Lung Cancer.Asian Pac J Cancer Prev. 2021 May 1;22(5):1495-1506. doi: 10.31557/APJCP.2021.22.5.1495. Asian Pac J Cancer Prev. 2021. PMID: 34048178 Free PMC article.
-
MAGE genes: Prognostic indicators in AL amyloidosis patients.J Cell Mol Med. 2019 Aug;23(8):5672-5678. doi: 10.1111/jcmm.14475. Epub 2019 Jun 20. J Cell Mol Med. 2019. PMID: 31222935 Free PMC article.
References
-
- Kyle RA, Linos A, Beard CM, Linke RP, Gertz MA, O'Fallon WM, et al. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood. 1992;79:1817–1822. - PubMed
-
- Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med. 1997;337:898–909. - PubMed
-
- Kyle RA, Gertz MA. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol. 1995;32:45–59. - PubMed
-
- Gertz MA, Zeldenrust SR. Treatment of immunoglobulin light chain amyloidosis. Curr Hematol Malig Rep. 2009;4:91–98. - PubMed
-
- Seldin DC, Choufani EB, Dember LM, Wiesman JF, Berk JL, Falk RH, et al. Tolerability and efficacy of thalidomide for the treatment of patients with light chain-associated (AL) amyloidosis. Clin Lymphoma. 2003;3:241–246. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

