Protein kinase D-HDAC5 signaling regulates erythropoiesis and contributes to erythropoietin cross-talk with GATA1
- PMID: 22983445
- PMCID: PMC3501719
- DOI: 10.1182/blood-2011-10-387050
Protein kinase D-HDAC5 signaling regulates erythropoiesis and contributes to erythropoietin cross-talk with GATA1
Abstract
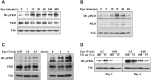
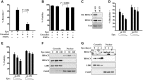
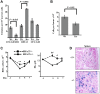
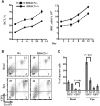
In red cell development, the differentiation program directed by the transcriptional regulator GATA1 requires signaling by the cytokine erythropoietin, but the mechanistic basis for this signaling requirement has remained unknown. Here we show that erythropoietin regulates GATA1 through protein kinase D activation, promoting histone deacetylase 5 (HDAC5) dissociation from GATA1, and subsequent GATA1 acetylation. Mice deficient for HDAC5 show resistance to anemic challenge and altered marrow responsiveness to erythropoietin injections. In ex vivo studies, HDAC5(-/-) progenitors display enhanced entry into and passage through the erythroid lineage, as well as evidence of erythropoietin-independent differentiation. These results reveal a molecular pathway that contributes to cytokine regulation of hematopoietic differentiation and offer a potential mechanism for fine tuning of lineage-restricted transcription factors by lineage-specific cytokines.
Figures


Similar articles
-
Identification of NuRSERY, a new functional HDAC complex composed by HDAC5, GATA1, EKLF and pERK present in human erythroid cells.Int J Biochem Cell Biol. 2014 May;50:112-22. doi: 10.1016/j.biocel.2014.02.019. Epub 2014 Mar 1. Int J Biochem Cell Biol. 2014. PMID: 24594363 Free PMC article.
-
Activation of phosphatidylinositol 3-kinase is important for erythropoietin-induced erythropoiesis from CD34(+) hematopoietic progenitor cells.Exp Hematol. 2002 Sep;30(9):990-1000. doi: 10.1016/s0301-472x(02)00868-8. Exp Hematol. 2002. PMID: 12225790
-
Erythropoietin stimulates phosphorylation and activation of GATA-1 via the PI3-kinase/AKT signaling pathway.Blood. 2006 Feb 1;107(3):907-15. doi: 10.1182/blood-2005-06-2516. Epub 2005 Oct 4. Blood. 2006. PMID: 16204311 Free PMC article.
-
[Erythropoiesis: a paradigm for the role of caspases in cell death and differentiation].J Soc Biol. 2005;199(3):219-31. doi: 10.1051/jbio:2005023. J Soc Biol. 2005. PMID: 16471262 Review. French.
-
Cellular mechanism of resistance to erythropoietin.Nephrol Dial Transplant. 1995;10 Suppl 6:27-30. doi: 10.1093/ndt/10.supp6.27. Nephrol Dial Transplant. 1995. PMID: 8524490 Review.
Cited by
-
Lysine Acetylation Goes Global: From Epigenetics to Metabolism and Therapeutics.Chem Rev. 2018 Feb 14;118(3):1216-1252. doi: 10.1021/acs.chemrev.7b00181. Epub 2018 Feb 6. Chem Rev. 2018. PMID: 29405707 Free PMC article. Review.
-
Histone Deacetylases Function in the Control of Early Hematopoiesis and Erythropoiesis.Int J Mol Sci. 2022 Aug 29;23(17):9790. doi: 10.3390/ijms23179790. Int J Mol Sci. 2022. PMID: 36077192 Free PMC article. Review.
-
The antidepressant action of 3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid is mediated by phosphorylation of histone deacetylase 5.Korean J Physiol Pharmacol. 2018 Mar;22(2):155-162. doi: 10.4196/kjpp.2018.22.2.155. Epub 2018 Feb 23. Korean J Physiol Pharmacol. 2018. PMID: 29520168 Free PMC article.
-
A time frame permissive for Protein Kinase D2 activity to direct angiogenesis in mouse embryonic stem cells.Sci Rep. 2015 Jul 7;5:11742. doi: 10.1038/srep11742. Sci Rep. 2015. PMID: 26148697 Free PMC article.
-
Identification of NuRSERY, a new functional HDAC complex composed by HDAC5, GATA1, EKLF and pERK present in human erythroid cells.Int J Biochem Cell Biol. 2014 May;50:112-22. doi: 10.1016/j.biocel.2014.02.019. Epub 2014 Mar 1. Int J Biochem Cell Biol. 2014. PMID: 24594363 Free PMC article.
References
-
- Gregory T, Yu C, Ma A, Orkin SH, Blobel GA, Weiss MJ. GATA-1 and erythropoietin cooperate to promote erythroid cell survival by regulating bcl-xL expression. Blood. 1999;94(1):87–96. - PubMed
-
- Ribeil J-A, Zermati Y, Vandekerckhove J, et al. Hsp70 regulates erythropoiesis by preventing caspase-3–mediated cleavage of GATA-1. Nature. 2007;445(1):102–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

