Reporting and analysis of trials using stratified randomisation in leading medical journals: review and reanalysis
- PMID: 22983531
- PMCID: PMC3444136
- DOI: 10.1136/bmj.e5840
Reporting and analysis of trials using stratified randomisation in leading medical journals: review and reanalysis
Abstract
Objectives: To assess how often stratified randomisation is used, whether analysis adjusted for all balancing variables, and whether the method of randomisation was adequately reported, and to reanalyse a previously reported trial to assess the impact of ignoring balancing factors in the analysis.
Design: Review of published trials and reanalysis of a previously reported trial.
Setting: Four leading general medical journals (BMJ, Journal of the American Medical Association, Lancet, and New England Journal of Medicine) and the second Multicenter Intrapleural Sepsis Trial (MIST2).
Participants: 258 trials published in 2010 in the four journals. Cluster randomised, crossover, non-randomised, single arm, and phase I or II trials were excluded, as were trials reporting secondary analyses, interim analyses, or results that had been previously published in 2010.
Main outcome measures: Whether the method of randomisation was adequately reported, how often balanced randomisation was used, and whether balancing factors were adjusted for in the analysis.
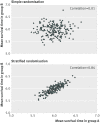
Results: Reanalysis of MIST2 showed that an unadjusted analysis led to larger P values and a loss of power. The review of published trials showed that balanced randomisation was common, with 163 trials (63%) using at least one balancing variable. The most common methods of balancing were stratified permuted blocks (n=85) and minimisation (n=27). The method of randomisation was unclear in 37% of trials. Most trials that balanced on centre or prognostic factors were not adequately analysed; only 26% of trials adjusted for all balancing factors in their primary analysis. Trials that did not adjust for balancing factors in their analysis were less likely to show a statistically significant result (unadjusted 57% v adjusted 78%, P=0.02).
Conclusion: Balancing on centre or prognostic factors is common in trials but often poorly described, and the implications of balancing are poorly understood. Trialists should adjust their primary analysis for balancing factors to obtain correct P values and confidence intervals and to avoid an unnecessary loss in power.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures
References
-
- Matts JP, Lachin JM. Properties of permuted-block randomization in clinical trials. Control Clin Trials 1988;9:327-44. - PubMed
-
- ICH Harmonised Tripartite Guideline. Statistical principles for clinical trials. International Conference on Harmonisation E9 Expert Working Group. Stat Med 1999;18:1905-42. - PubMed
-
- Kahan BC, Morris TP. Improper analysis of trials randomised using stratified blocks or minimisation. Stat Med 2012;31:328-40. - PubMed
-
- Kernan WN, Viscoli CM, Makuch RW, Brass LM, Horwitz RI. Stratified randomization for clinical trials. J Clin Epidemiol 1999;52:19-26. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical