Single-agent therapy with sorafenib or 5-FU is equally effective in human colorectal cancer xenograft--no benefit of combination therapy
- PMID: 22983756
- PMCID: PMC3587684
- DOI: 10.1007/s00384-012-1551-2
Single-agent therapy with sorafenib or 5-FU is equally effective in human colorectal cancer xenograft--no benefit of combination therapy
Abstract
Background: We initiated this preclinical study in order to analyze the impact of sorafenib single treatment versus combination treatment in human colorectal cancer.
Methods: The effect of increasing sorafenib doses on proliferation, apoptosis, migration, and activation of signal cascades was analyzed in vitro. The effect of sorafenib single treatment versus 5-fluorouracil (5-FU) single treatment and combination therapy on in vivo proliferation and target cytokine receptor/ligand expression was analyzed in a human colon cancer xenograft mouse model using HT29 tumor cells.
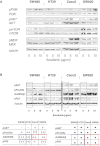
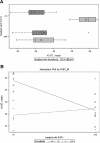
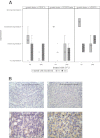
Results: In vitro, SW480 and HT29 cell lines were sensitive to sorafenib, as compared to Caco2 and SW620 cell lines, independent of the mutation status of K-ras, Raf, PTEN, or PI3K. The effect on migration was marginal, but distinct differences in caspases activation were seen. Combination strategies were beneficial in some settings (sorafenib + 5-FU; irinotecan) and disadvantageous in others (sorafenib + oxaliplatin), depending on the chemotherapeutic drug and cell line chosen. Sensitive cell lines revealed a downregulation of AKT and had a weak expression level of GADD45β. In resistant cell lines, pp53 and GADD45β levels decreased upon sorafenib exposure. In vivo, the combination treatment of sorafenib and 5-FU was equally effective as the respective monotherapy concerning tumor proliferation. Interestingly, treatment with either sorafenib or 5-FU resulted in a significant decrease of VEGFR1 and PDGFRβ expression intensity.
Conclusions: In colorectal cancer, a sensitivity towards sorafenib exists, which seems similarly effective as a 5-FU monotherapy. A combination therapy, in contrast, does not show any additional effect.
Figures


References
-
- Weir HK, Thun MJ, Hankey BF, Ries LA, Howe HL, Wingo PA, Jemal A, Ward E, Anderson RN, Edwards BK. Annual report to the nation on the status of cancer, 1975–2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst. 2003;95:1276–1299. doi: 10.1093/jnci/djg040. - DOI - PubMed
-
- Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocakova I, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28(31):4697–7705. doi: 10.1200/JCO.2009.27.4860. - DOI - PubMed
-
- Sobrero A, Ackland S, Clarke S, Perez-Carrion R, Chiara S, Gapski J, Mainwaring P, Langer B, Young S. Phase IV study of bevacizumab in combination with infusional fluorouracil, leucovorin and irinotecan (FOLFIRI) in first-line metastatic colorectal cancer. Oncology. 2009;77:113–119. doi: 10.1159/000229787. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

