Retroviral-vector-mediated gene therapy to mucopolysaccharidosis I mice improves sensorimotor impairments and other behavioral deficits
- PMID: 22983812
- PMCID: PMC3548941
- DOI: 10.1007/s10545-012-9530-x
Retroviral-vector-mediated gene therapy to mucopolysaccharidosis I mice improves sensorimotor impairments and other behavioral deficits
Abstract
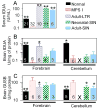
Mucopolysaccharidosis I (MPS I) is a lysosomal storage disease due to α-L-iduronidase (IDUA) deficiency that results in the accumulation of glycosaminoglycans (GAG). Systemic gene therapy to MPS I mice can reduce lysosomal storage in the brain, but few data are available regarding the effect upon behavioral function. We investigated the effect of gene therapy with a long-terminal-repeat (LTR)-intact retroviral vector or a self-inactivating (SIN) vector on behavioral function in MPS I mice. The LTR vector was injected intravenously to 6-week-old MPS I mice, and the SIN vector was given to neonatal or 6-week-old mice. Adult-LTR, neonatal-SIN, and adult-SIN-treated mice achieved serum IDUA activity of 235 ± 20 (84-fold normal), 127 ± 10, and 71 ± 7 U/ml, respectively. All groups had reduction in histochemical evidence of lysosomal storage in the brain, with the adult-LTR group showing the best response, while adult-LTR mice had reductions in lysosomal storage in the cristae of the vestibular system. Behavioral evaluation was performed at 8 months. Untreated MPS I mice had a markedly reduced ability to hold onto an inverted screen or climb down a pole. LTR-vector-treated mice had marked improvements on both of these tests, whereas neonatal-SIN mice showed improvement in the pole test. We conclude that both vectors can reduce brain disease in MPS I mice, with the LTR vector achieving higher serum IDUA levels and better correction. Vestibular abnormalities may contribute to mobility problems in patients with MPS I, and gene therapy may reduce symptoms.
Figures
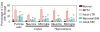




Similar articles
-
A self-inactivating gamma-retroviral vector reduces manifestations of mucopolysaccharidosis I in mice.Mol Ther. 2010 Feb;18(2):334-42. doi: 10.1038/mt.2009.236. Epub 2009 Oct 20. Mol Ther. 2010. PMID: 19844196 Free PMC article.
-
Improved retroviral vector design results in sustained expression after adult gene therapy in mucopolysaccharidosis I mice.J Gene Med. 2008 Sep;10(9):972-82. doi: 10.1002/jgm.1229. J Gene Med. 2008. PMID: 18613275 Free PMC article.
-
Correction of clinical manifestations of canine mucopolysaccharidosis I with neonatal retroviral vector gene therapy.Mol Ther. 2007 Aug;15(8):1423-31. doi: 10.1038/sj.mt.6300201. Epub 2007 May 22. Mol Ther. 2007. PMID: 17519893
-
Mucopolysaccharidoses type I gene therapy.J Inherit Metab Dis. 2021 Sep;44(5):1088-1098. doi: 10.1002/jimd.12414. Epub 2021 Jul 9. J Inherit Metab Dis. 2021. PMID: 34189746 Free PMC article. Review.
-
Murine mucopolysaccharidosis type VII: the impact of therapies on the clinical course and pathology in a murine model of lysosomal storage disease.J Inherit Metab Dis. 1998 Aug;21(5):575-86. doi: 10.1023/a:1005423222927. J Inherit Metab Dis. 1998. PMID: 9728337 Review.
Cited by
-
Efficacy of a Combination Therapy with Laronidase and Genistein in Treating Mucopolysaccharidosis Type I in a Mouse Model.Int J Mol Sci. 2024 Feb 17;25(4):2371. doi: 10.3390/ijms25042371. Int J Mol Sci. 2024. PMID: 38397051 Free PMC article.
-
Therapies of mucopolysaccharidosis IVA (Morquio A syndrome).Expert Opin Orphan Drugs. 2013 Oct 1;1(10):805-818. doi: 10.1517/21678707.2013.846853. Expert Opin Orphan Drugs. 2013. PMID: 25419501 Free PMC article.
-
Lysosomal storage disease: gene therapy on both sides of the blood-brain barrier.Mol Genet Metab. 2015 Feb;114(2):83-93. doi: 10.1016/j.ymgme.2014.09.011. Epub 2014 Oct 7. Mol Genet Metab. 2015. PMID: 25410058 Free PMC article. Review.
-
Neonatal cellular and gene therapies for mucopolysaccharidoses: the earlier the better?J Inherit Metab Dis. 2016 Mar;39(2):189-202. doi: 10.1007/s10545-015-9900-2. Epub 2015 Nov 17. J Inherit Metab Dis. 2016. PMID: 26578156 Free PMC article. Review.
-
Mucopolysaccharidosis IVA: Diagnosis, Treatment, and Management.Int J Mol Sci. 2020 Feb 23;21(4):1517. doi: 10.3390/ijms21041517. Int J Mol Sci. 2020. PMID: 32102177 Free PMC article. Review.
References
-
- Aldenhoven M, Boelens JJ, de Koning TJ. The Clinical Outcome of Hurler Syndrome after Stem Cell Transplantation. Biology of Blood and Marrow Transplantation. 2008;14:485–498. - PubMed
-
- Baehner F, Schmiedeskamp C, Krummenauer F, et al. Cumulative incidence rates of the mucopolysaccharidoses in Germany. J Inherit Metab Dis. 2005;28:1011–1017. - PubMed
-
- Banks WA. Are the extracellular pathways a conduit for the delivery of therapeutics to the brain. Curr Pharm Des. 2004;10:1365–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

