Structural basis of metal hypersensitivity
- PMID: 22983897
- PMCID: PMC4040395
- DOI: 10.1007/s12026-012-8351-1
Structural basis of metal hypersensitivity
Abstract
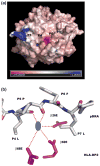
Metal hypersensitivity is a common immune disorder. Human immune systems mount the allergic attacks on metal ions through skin contacts, lung inhalation and metal-containing artificial body implants. The consequences can be simple annoyances to life-threatening systemic illness. Allergic hyper-reactivities to nickel (Ni) and beryllium (Be) are the best-studied human metal hypersensitivities. Ni-contact dermatitis affects 10 % of the human population, whereas Be compounds are the culprits of chronic Be disease (CBD). αβ T cells (T cells) play a crucial role in these hypersensitivity reactions. Metal ions work as haptens and bind to the surface of major histocompatibility complex (MHC) and peptide complex. This modifies the binding surface of MHC and triggers the immune response of T cells. Metal-specific αβ T cell receptors (TCRs) are usually MHC restricted, especially MHC class II (MHCII) restricted. Numerous models have been proposed, yet the mechanisms and molecular basis of metal hypersensitivity remain elusive. Recently, we determined the crystal structures of the Ni and Be presenting human MHCII molecules, HLA-DR52c (DRA*0101, DRB3*0301) and HLA-DP2 (DPA1*0103, DPB1*0201). These structures revealed unusual features of MHCII molecules and shed light on how metal ions are recognized by T cells.
Figures


References
-
- Budinger L, Hertl M. Immunologic mechanisms in hypersensitivity reactions to metal ions: an overview. Allergy. 2000;55(2):108–15. - PubMed
-
- Schiraldi M, Monestier M. How can a chemical element elicit complex immunopathology? Lessons from mercury-induced autoimmunity. Trends Immunol. 2009;30(10):502–9. - PubMed
-
- Vas J, Monestier M. Immunology of mercury. Ann N Y Acad Sci. 2008;1143:240–67. - PubMed
-
- Rowley B, Monestier M. Mechanisms of heavy metal-induced autoimmunity. Mol Immunol. 2005;42(7):833–8. - PubMed
-
- Li H, Llera A, Malchiodi EL, Mariuzza RA. The structural basis of T cell activation by superantigens. Annu Rev Immunol. 1999;17:435–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

