Pantothenate kinase-associated neurodegeneration: altered mitochondria membrane potential and defective respiration in Pank2 knock-out mouse model
- PMID: 22983956
- PMCID: PMC3510755
- DOI: 10.1093/hmg/dds380
Pantothenate kinase-associated neurodegeneration: altered mitochondria membrane potential and defective respiration in Pank2 knock-out mouse model
Abstract
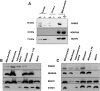
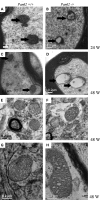
Neurodegeneration with brain iron accumulation (NBIA) comprises a group of neurodegenerative disorders characterized by high brain content of iron and presence of axonal spheroids. Mutations in the PANK2 gene, which encodes pantothenate kinase 2, underlie an autosomal recessive inborn error of coenzyme A metabolism, called pantothenate kinase-associated neurodegeneration (PKAN). PKAN is characterized by dystonia, dysarthria, rigidity and pigmentary retinal degeneration. The pathogenesis of this disorder is poorly understood and, although PANK2 is a mitochondrial protein, perturbations in mitochondrial bioenergetics have not been reported. A knock-out (KO) mouse model of PKAN exhibits retinal degeneration and azoospermia, but lacks any neurological phenotype. The absence of a clinical phenotype has partially been explained by the different cellular localization of the human and murine PANK2 proteins. Here we demonstrate that the mouse Pank2 protein localizes to mitochondria, similar to its human orthologue. Moreover, we show that Pank2-defective neurons derived from KO mice have an altered mitochondrial membrane potential, a defect further corroborated by the observations of swollen mitochondria at the ultra-structural level and by the presence of defective respiration.
Figures




References
-
- Spector R., Johanson C.E. Vitamin transport and homeostasis in mammalian brain: focus on Vitamins B and E. J. Neurochem. 2007;103:425–438. doi:10.1111/j.1471-4159.2007.04773.x. - DOI - PubMed
-
- Daugherty M., Polanuyer B., Farrell M., Scholle M., Lykidis A., de Crécy-Lagard V., Osterman A. Complete reconstitution of the human coenzyme A biosynthetic pathway via comparative genomics. J. Biol. Chem. 2002;277:21431–21439. doi:10.1074/jbc.M201708200. - DOI - PubMed
-
- Skrede S., Halvorsen O. Mitochondrial biosynthesis of coenzyme A. Biochem. Biophys. Res. Commun. 1979;91:1536–1542. doi:10.1016/0006-291X(79)91239-7. - DOI - PubMed
-
- Hörtnagel K., Prokisch H., Meitinger T. An isoform of hPANK2, deficient in pantothenate kinase-associated neurodegeneration, localizes to mitochondria. Hum. Mol. Genet. 2003;12:321–327. doi:10.1093/hmg/ddg026. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

