Protective immunity against pulmonary cryptococcosis is associated with STAT1-mediated classical macrophage activation
- PMID: 22984078
- PMCID: PMC3466339
- DOI: 10.4049/jimmunol.1103455
Protective immunity against pulmonary cryptococcosis is associated with STAT1-mediated classical macrophage activation
Abstract
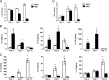
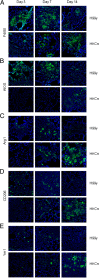
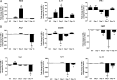
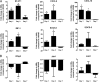
Experimental pulmonary Cryptococcus neoformans infection in BALB/c mice is associated with polarized Th2-type cytokine production, alternative macrophage activation, and severe bronchopneumonia. In contrast, pulmonary infection with a C. neoformans strain that secretes IFN-γ, H99γ, elicits Th1-type cytokine production and classical macrophage activation. Additionally, mice infected with H99γ resolve the acute infection and are subsequently protected against challenge with wild-type C. neoformans. The present study characterizes macrophage activation during the protective response to wild-type C. neoformans in mice previously immunized with H99γ. We observed increased pulmonary Th1-type cytokine production in lung homogenates and classical macrophage activation as evidenced by enhanced expression of inducible NO synthase in the lungs of H99γ-immunized mice compared with mice given a nonprotective immunization with heat-killed C. neoformans (HKCn). Furthermore, macrophages isolated from H99γ-immunized mice on day 7 postchallenge and cultured in vitro were fungistatic against C. neoformans, whereas cryptococcal growth was uncontrolled within macrophages from HKCn-immunized mice. Th2-type cytokine production and induction of alternatively activated macrophages were also observed in lungs of HKCn-immunized mice during rechallenge. Gene expression arrays showed that classical macrophage activation during challenge infection in H99γ-immunized mice was associated with induction of the transcription factor STAT1 and its downstream targets IFN regulatory factor-1, suppressor of cytokine signaling-1, CXCL9, and CXCL10. These studies demonstrate that protective responses to C. neoformans challenge in immunized mice include classical macrophage activation and enhanced macrophage fungistasis of C. neoformans yeasts. Finally, the classical activation phenotype of protective anticryptococcal macrophages is likely mediated via STAT1 signal transduction pathways.
Figures


References
-
- Park B. J., Wannemuehler K. A., Marston B. J., Govender N., Pappas P. G., Chiller T. M. 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23: 525–530 - PubMed
-
- Martinez F. O., Helming L., Gordon S. 2009. Alternative activation of macrophages: an immunologic functional perspective. Annu. Rev. Immunol. 27: 451–483 - PubMed
-
- Flesch I. E., Schwamberger G., Kaufmann S. H. 1989. Fungicidal activity of IFN-γ-activated macrophages: extracellular killing of Cryptococcus neoformans. J. Immunol. 142: 3219–3224 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

