Receptor cross-talk spatially restricts p-ERK during TLR4 stimulation of autoreactive B cells
- PMID: 22984080
- PMCID: PMC3466401
- DOI: 10.4049/jimmunol.1200940
Receptor cross-talk spatially restricts p-ERK during TLR4 stimulation of autoreactive B cells
Abstract
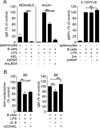
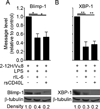
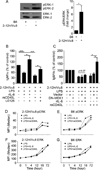
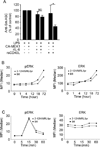
To maintain tolerance, autoreactive B cells must regulate signal transduction from the BCR and TLRs. We recently identified that dendritic cells and macrophages regulate autoreactive cells during TLR4 activation by releasing IL-6 and soluble CD40 ligand (sCD40L). These cytokines selectively repress Ab secretion from autoreactive, but not antigenically naive, B cells. How IL-6 and sCD40L repress autoantibody production is unknown. In this work, we show that IL-6 and sCD40L are required for low-affinity/avidity autoreactive B cells to maintain tolerance through a mechanism involving receptor cross-talk between the BCR, TLR4, and the IL-6R or CD40. We show that acute signaling through IL-6R or CD40 integrates with chronic BCR-mediated ERK activation to restrict p-ERK from the nucleus and represses TLR4-induced Blimp-1 and XBP-1 expression. Tolerance is disrupted in 2-12H/MRL/lpr mice where IL-6 and sCD40L fail to spatially restrict p-ERK and fail to repress TLR4-induced Ig secretion. In the case of CD40, acute signaling in B cells from 2-12H/MRL/lpr mice is intact, but the chronic activation of p-ERK emanating from the BCR is attenuated. Re-establishing chronically active ERK through retroviral expression of constitutively active MEK1 restores tolerance upon sCD40L, but not IL-6, stimulation, indicating that regulation by IL-6 requires another signaling effector. These data define the molecular basis for the regulation of low-affinity autoreactive B cells during TLR4 stimulation; they explain how autoreactive but not naive B cells are repressed by IL-6 and sCD40L; and they identify B cell defects in lupus-prone mice that lead to TLR4-induced autoantibody production.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no conflicting financial interests.
Figures


Similar articles
-
Autoreactive preplasma cells break tolerance in the absence of regulation by dendritic cells and macrophages.J Immunol. 2012 Jul 15;189(2):711-20. doi: 10.4049/jimmunol.1102973. Epub 2012 Jun 6. J Immunol. 2012. PMID: 22675201 Free PMC article.
-
Dendritic cells from lupus-prone mice are defective in repressing immunoglobulin secretion.J Immunol. 2007 Apr 15;178(8):4803-10. doi: 10.4049/jimmunol.178.8.4803. J Immunol. 2007. PMID: 17404261 Free PMC article.
-
B cell receptor cross-talk: exposure to lipopolysaccharide induces an alternate pathway for B cell receptor-induced ERK phosphorylation and NF-kappa B activation.J Immunol. 2007 Jul 1;179(1):229-35. doi: 10.4049/jimmunol.179.1.229. J Immunol. 2007. PMID: 17579042
-
B-cell receptor signaling in lymphoid malignancies and autoimmunity.Adv Immunol. 2014;123:1-49. doi: 10.1016/B978-0-12-800266-7.00004-2. Adv Immunol. 2014. PMID: 24840946 Review.
-
The role of B cells in lupus pathogenesis.Int J Biochem Cell Biol. 2010 Apr;42(4):543-50. doi: 10.1016/j.biocel.2009.10.011. Epub 2009 Oct 20. Int J Biochem Cell Biol. 2010. PMID: 19850148 Free PMC article. Review.
Cited by
-
Apoptotic Debris Accumulates on Hematopoietic Cells and Promotes Disease in Murine and Human Systemic Lupus Erythematosus.J Immunol. 2016 May 15;196(10):4030-9. doi: 10.4049/jimmunol.1500418. Epub 2016 Apr 8. J Immunol. 2016. PMID: 27059595 Free PMC article.
-
Roles of B Cell-Intrinsic TLR Signals in Systemic Lupus Erythematosus.Int J Mol Sci. 2015 Jun 9;16(6):13084-105. doi: 10.3390/ijms160613084. Int J Mol Sci. 2015. PMID: 26068236 Free PMC article. Review.
-
Altered toll-like receptor responsiveness underlies a dominant heritable defect in B cell tolerance in autoimmune New Zealand Black mice.Eur J Immunol. 2018 Mar;48(3):492-497. doi: 10.1002/eji.201747287. Epub 2018 Jan 19. Eur J Immunol. 2018. PMID: 29251774 Free PMC article.
-
Staphylococcus aureus Protein A Disrupts Immunity Mediated by Long-Lived Plasma Cells.J Immunol. 2017 Feb 1;198(3):1263-1273. doi: 10.4049/jimmunol.1600093. Epub 2016 Dec 28. J Immunol. 2017. PMID: 28031339 Free PMC article.
-
Silica Exposure Differentially Modulates Autoimmunity in Lupus Strains and Autoantibody Transgenic Mice.Front Immunol. 2019 Oct 1;10:2336. doi: 10.3389/fimmu.2019.02336. eCollection 2019. Front Immunol. 2019. PMID: 31632407 Free PMC article.
References
-
- Ferry H, Leung JC, Lewis G, Nijnik A, Silver K, Lambe T, et al. B-cell tolerance. Transplantation. 2006;81:308–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

