Comparative effectiveness of a rapid point-of-care test for detection of Chlamydia trachomatis among women in a clinical setting
- PMID: 22984085
- PMCID: PMC3671871
- DOI: 10.1136/sextrans-2011-050355
Comparative effectiveness of a rapid point-of-care test for detection of Chlamydia trachomatis among women in a clinical setting
Abstract
Objectives: To compare the effectiveness and cost-effectiveness of a promising new point-of-care (POC) chlamydia test with traditional nucleic acid amplification testing (NAAT), and to determine the characteristics that would make a POC test most cost-effective.
Methods: A decision tree was constructed to model chlamydia screening visits to a sexually transmitted disease clinic by a hypothetical cohort of 10,000 women. The model incorporated programmatic screening costs, treatment costs and medical costs averted through prevention of pelvic inflammatory disease (PID) and its sequelae. Parameter values and costs were estimated for each node in the decision tree based on primary data, published data and unpublished health data.
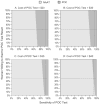
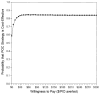
Results: For the base-case scenario (POC sensitivity 92.9%; 47.5% of women willing to wait 40 min for test results; test cost $33.48), POC was estimated to save US$5050 for each case of PID averted compared with NAAT. One-way sensitivity analyses indicated that POC would dominate NAAT if the POC test cost is <US$41.52 or if POC sensitivity is ≥ 87.1%. In a probabilistic sensitivity analysis (Monte Carlo simulations, 10 000 iterations), 10.8% of iterations indicated that the POC strategy dominated the NAAT strategy. The mean incremental cost-effectiveness ratio indicated that the POC strategy would save US$28 in total, and avert 14 PID cases.
Conclusions: A promising new chlamydia POC test is likely to be cost-effective compared with traditional NAAT. The POC test sensitivity, cost and proportion of women willing to wait for the POC test result are key elements to determining the cost-effectiveness of any new POC test strategy.
Conflict of interest statement
Figures
References
-
- Centers for Disease Control and Prevention (CDC) Chlamydia screening among sexually active young female enrollees of health plans—United States, 2000–2007. MMWR Morb Mortal Wkly Rep. 2009;58:362–5. - PubMed
-
- Gift TL, Pate MS, Hook EW, III, et al. The rapid test paradox: when fewer cases detected lead to more cases treated: a decision analysis of tests for Chlamydia trachomatis. Sex Transm Dis. 1999;26:232–40. - PubMed
-
- Lau C-Y, Qureshi AK. Azithromycin versus doxycycline for genital chlamydial infections: a meta-analysis of randomized clinical trials. Sex Transm Dis. 2002;29:497–502. - PubMed
-
- Schwebke JR, Sadler R, Sutton JM, et al. Positive screening tests for gonorrhea and chlamydial infection fail to lead consistently to treatment of patients attending a sexually transmitted disease clinic. Sex Transm Dis. 1997;24:181–4. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
