Response to raltegravir-based salvage therapy in HIV-infected patients with hepatitis C virus or hepatitis B virus coinfection
- PMID: 22984206
- PMCID: PMC3522446
- DOI: 10.1093/jac/dks341
Response to raltegravir-based salvage therapy in HIV-infected patients with hepatitis C virus or hepatitis B virus coinfection
Abstract
Objectives: To define the impact of coinfection with hepatitis B virus (HBV) or hepatitis C virus (HCV) on viroimmunological response to raltegravir-based salvage regimens that also include new HIV inhibitors such as maraviroc, darunavir and etravirine.
Methods: We used data from a national observational study of patients starting raltegravir-based regimens to compare virological suppression and CD4 cell change from baseline in patients with and without concomitant HBV or HCV infection.
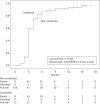
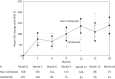
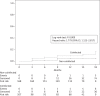
Results: Overall, 275 patients (107 coinfected and 168 non-coinfected) were evaluated. Coinfected patients were more commonly former intravenous drug users and had a longer history of HIV infection and higher baseline aminotransferase levels. Both HIV-RNA and CD4 response were similar in the two groups. Mean time to first HIV-RNA copy number <50 copies/mL was 4.1 months (95% CI 3.5-4.6) in non-coinfected patients and 3.9 months (95% CI 3.3-4.5) in coinfected patients (hazard ratio 1.039, 95% CI 0.761-1.418, P = 0.766, log-rank test). The risk of developing new grade 3-4 hepatic adverse events was significantly higher in coinfected patients (hazard ratio 1.779, 95% CI 1.123-2.817, P = 0.009). The two groups of coinfected and non-coinfected patients had similar rates of interruption of any baseline drug (hazard ratio 1.075, 95% CI 0.649-1.781, P = 0.776) and of raltegravir (hazard ratio 1.520, 95% CI 0.671-3.447, P = 0.311). Few AIDS-defining events and deaths occurred.
Conclusions: Viroimmunological response to regimens based on raltegravir and other recent anti-HIV inhibitors is not negatively affected by coinfection with HBV or HCV. Liver toxicity, either pre-existing or new, is more common in coinfected patients, but with no increased risk of treatment interruption.
Figures
References
-
- Rockstroh JK, Mocroft A, Soriano V, et al. Influence of hepatitis C virus infection on HIV-1 disease progression and response to highly active antiretroviral therapy. J Infect Dis. 2005;192:992–1002. - PubMed
-
- Yacisin K, Maida I, Ríos MJ, et al. Hepatitis C virus coinfection does not affect CD4 restoration in HIV-infected patients after initiation of antiretroviral therapy. AIDS Res Hum Retroviruses. 2008;24:935–40. - PubMed
-
- Fuping G, Wei L, Yang H, et al. Impact of hepatitis C virus coinfection on HAART in HIV-infected individuals: multicentric observation cohort. J Acquir Immune Defic Syndr. 2010;54:137–42. - PubMed
-
- Carmo RA, Guimarães MD, Moura AS, et al. The influence of HCV coinfection on clinical, immunological and virological responses to HAART in HIV-patients. Braz J Infect Dis. 2008;12:173–9. - PubMed
-
- Weis N, Lindhardt BO, Kronborg G, et al. Impact of hepatitis C virus coinfection on response to highly active antiretroviral therapy and outcome in HIV-infected individuals: a nationwide cohort study. Clin Infect Dis. 2006;42:1481–7. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

