Endothelial γ-glutamyltransferase contributes to the vasorelaxant effect of S-nitrosoglutathione in rat aorta
- PMID: 22984412
- PMCID: PMC3439434
- DOI: 10.1371/journal.pone.0043190
Endothelial γ-glutamyltransferase contributes to the vasorelaxant effect of S-nitrosoglutathione in rat aorta
Abstract
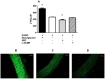
S-nitrosoglutathione (GSNO) involved in storage and transport of nitric oxide ((•)NO) plays an important role in vascular homeostasis. Breakdown of GSNO can be catalyzed by γ-glutamyltransferase (GGT). We investigated whether vascular GGT influences the vasorelaxant effect of GSNO in isolated rat aorta. Histochemical localization of GGT and measurement of its activity were performed by using chromogenic substrates in sections and in aorta homogenates, respectively. The role of GGT in GSNO metabolism was evaluated by measuring GSNO consumption rate (absorbance decay at 334 nm), (•)NO release was visualized and quantified with the fluorescent probe 4,5-diaminofluorescein diacetate. The vasorelaxant effect of GSNO was assayed using isolated rat aortic rings (in the presence or absence of endothelium). The role of GGT was assessed by stimulating enzyme activity with cosubstrate glycylglycine, as well as using two independent inhibitors, competitive serine borate complex and non-competitive acivicin. Specific GGT activity was histochemically localized in the endothelium. Consumption of GSNO and release of free (•)NO decreased and increased in presence of serine borate complex and glycylglycine, respectively. In vasorelaxation experiments with endothelium-intact aorta, the half maximal effective concentration of GSNO (EC50 = 3.2 ± 0.5.10(-7) M) increased in the presence of the two distinct GGT inhibitors, serine borate complex (1.6 ± 0.2.10(-6) M) and acivicin (8.3 ± 0.6.10(-7) M), while it decreased with glycylglycine (4.7 ± 0.9.10(-8) M). In endothelium-denuded aorta, EC(50) for GSNO alone increased to 2.3 ± 0.3.10(-6) M, with no change in the presence of serine borate complex. These data demonstrate the important role of endothelial GGT activity in mediating the vasorelaxant effect of GSNO in rat aorta under physiological conditions. Because therapeutic treatments based on GSNO are presently under development, this endothelium-dependent mechanism involved in the vascular effects of GSNO should be taken into account in a pharmacological perspective.
Conflict of interest statement
Figures



References
-
- Krejcy K, Schmetterer L, Kastner J, Nieszpaur-Los M, Monitzer B, et al. (1995) Role of nitric oxide in hemostatic system activation in vivo in humans. Arterioscler Thromb Vasc Biol 15: 2063–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

