Fasting enhances the response of glioma to chemo- and radiotherapy
- PMID: 22984531
- PMCID: PMC3439413
- DOI: 10.1371/journal.pone.0044603
Fasting enhances the response of glioma to chemo- and radiotherapy
Abstract
Background: Glioma, including anaplastic astrocytoma and glioblastoma multiforme (GBM) are among the most commonly diagnosed malignant adult brain tumors. GBM is a highly invasive and angiogenic tumor, resulting in a 12 to 15 months median survival. The treatment of GBM is multimodal and includes surgical resection, followed by adjuvant radio-and chemotherapy. We have previously reported that short-term starvation (STS) enhances the therapeutic index of chemo-treatments by differentially protecting normal cells against and/or sensitizing tumor cells to chemotoxicity.
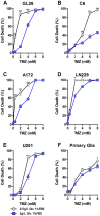
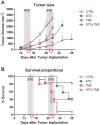
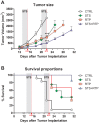
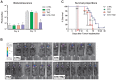
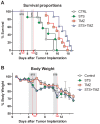
Methodology and principal findings: To test the effect of starvation on glioma cells in vitro, we treated primary mouse glia, murine GL26, rat C6 and human U251, LN229 and A172 glioma cells with Temozolomide in ad lib and STS mimicking conditions. In vivo, mice with subcutaneous or intracranial models of GL26 glioma were starved for 48 hours prior to radio- or chemotherapy and the effects on tumor progression and survival were measured. Starvation-mimicking conditions sensitized murine, rat and human glioma cells, but not primary mixed glia, to chemotherapy. In vivo, starvation for 48 hours, which causes a significant reduction in blood glucose and circulating insulin-like growth factor 1 (IGF-1) levels, sensitized both subcutaneous and intracranial glioma models to radio-and chemotherapy.
Conclusion: Starvation-induced cancer sensitization to radio- or chemotherapy leads to extended survival in the in vivo glioma models tested. These results indicate that fasting and fasting-mimicking interventions could enhance the efficacy of existing cancer treatments against aggressive glioma in patients.
Conflict of interest statement
Figures
References
-
- Ohgaki H, Kleihues P (2005) Epidemiology and etiology of gliomas. Acta Neuropathol 109: 93–108. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, et al. (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987–996. - PubMed
-
- Stupp R, Dietrich PY, Ostermann Kraljevic S, Pica A, Maillard I, et al. (2002) Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol 20: 1375–1382. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

