A novel stress-induced sugarcane gene confers tolerance to drought, salt and oxidative stress in transgenic tobacco plants
- PMID: 22984543
- PMCID: PMC3439409
- DOI: 10.1371/journal.pone.0044697
A novel stress-induced sugarcane gene confers tolerance to drought, salt and oxidative stress in transgenic tobacco plants
Abstract
Background: Drought is a major abiotic stress that affects crop productivity worldwide. Sugarcane can withstand periods of water scarcity during the final stage of culm maturation, during which sucrose accumulation occurs. Meanwhile, prolonged periods of drought can cause severe plant losses.
Methodology/principal findings: In a previous study, we evaluated the transcriptome of drought-stressed plants to better understand sugarcane responses to drought. Among the up-regulated genes was Scdr1 (sugarcane drought-responsive 1). The aim of the research reported here was to characterize this gene. Scdr1 encodes a putative protein containing 248 amino acids with a large number of proline (19%) and cysteine (13%) residues. Phylogenetic analysis showed that ScDR1is in a clade with homologs from other monocotyledonous plants, separate from those of dicotyledonous plants. The expression of Scdr1 in different varieties of sugarcane plants has not shown a clear association with drought tolerance.
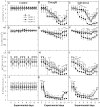
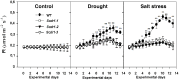
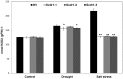
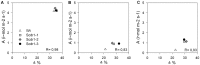
Conclusions/significance: The overexpression of Scdr1 in transgenic tobacco plants increased their tolerance to drought, salinity and oxidative stress, as demonstrated by increased photosynthesis, water content, biomass, germination rate, chlorophyll content and reduced accumulation of ROS. Physiological parameters, such as transpiration rate (E), net photosynthesis (A), stomatal conductance (gs) and internal leaf CO(2) concentration, were less affected by abiotic stresses in transgenic Scdr1 plants compared with wild-type plants. Overall, our results indicated that Scdr1 conferred tolerance to multiple abiotic stresses, highlighting the potential of this gene for biotechnological applications.
Conflict of interest statement
Figures








References
-
- Lawlor DW, Cornic G (2002) Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ 25: 275–294. - PubMed
-
- Boyer JS (1982) Plant productivity and environment. Science 218: 443–448. - PubMed
-
- Maybank J, Bonsal BR, Jones J, Lawford RG, O’Brien EG, et al. (1995) Drought as a natural disaster. Atmosphere-Ocean 33: 195–222.
-
- Solari LI, Johnson S, DeJong TM (2006) Relationship of water status to vegetative growth and leaf gas exchange of peach (Prunus persica) trees on different rootstocks. Tree Physiol 26: 1333–1341. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

