Sex differences in carbohydrate metabolism are linked to gene expression in Caenorhabditis elegans
- PMID: 22984551
- PMCID: PMC3439400
- DOI: 10.1371/journal.pone.0044748
Sex differences in carbohydrate metabolism are linked to gene expression in Caenorhabditis elegans
Abstract
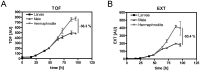
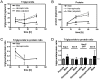
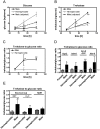
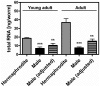
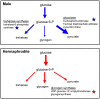
The male and the hermaphrodite forms of the nematode Caenorhabditis elegans (C. elegans) differ markedly in anatomy, nervous system and behavior at adulthood. Using the male mutants fog-2, him-5, and him-8, we compared body proportions and composition, and aspects of carbohydrate metabolism and gene expression between the C. elegans sexes in three adult stages. In all experiments, both sexes were grown on the same plate and separated using flow cytometry. The fat to fat-free mass ratio and the body volume-adjusted fat mass is similar between the sexes, although the body size is more than 50% smaller in adult males than in age-matched hermaphrodites. The volume-adjusted total RNA content is approximately 2-fold lower in males. Biochemical and NMR-based analyses reveal higher trehalose levels and much lower glucose levels in males than in hermaphrodites. The resulting trehalose-to-glucose ratio is 5.4-fold higher in males. These sex differences are reflected in gene expression data because the genes encoding key enzymes of the glycolysis and trehalose synthesis pathways are more highly expressed in males than in hermaphrodites. Notably, expression of the phosphofructokinase gene (C50F4.2) is 29-fold higher in males. Comparative analysis of gene expression data identifies 285 male-specific and 160 hermaphrodite-specific genes. These include transcription factor and C-type lectin-encoding genes. More than 35% of all C-type lectin genes are more highly expressed in males. The expression of many C-type lectin genes differs by a factor of >100 between the sexes. In conclusion, we found sex differences in carbohydrate metabolism that are linked to gene expression and identified certain lectin genes that are differentially expressed by the C. elegans sexes.
Conflict of interest statement
Figures





Similar articles
-
Sex differences in body composition, fat storage, and gene expression profile in Caenorhabditis elegans in response to dietary restriction.Physiol Genomics. 2013 Jul 2;45(13):539-51. doi: 10.1152/physiolgenomics.00007.2013. Epub 2013 May 28. Physiol Genomics. 2013. PMID: 23715261
-
Trehalose metabolism genes in Caenorhabditis elegans and filarial nematodes.Int J Parasitol. 2003 Sep 30;33(11):1195-206. doi: 10.1016/s0020-7519(03)00173-5. Int J Parasitol. 2003. PMID: 13678635 Review.
-
Somatic sexual differentiation in Caenorhabditis elegans.Curr Top Dev Biol. 2008;83:1-39. doi: 10.1016/S0070-2153(08)00401-8. Curr Top Dev Biol. 2008. PMID: 19118662
-
Dynamic, Non-binary Specification of Sexual State in the C. elegans Nervous System.Curr Biol. 2020 Sep 21;30(18):3617-3623.e3. doi: 10.1016/j.cub.2020.07.007. Epub 2020 Jul 23. Curr Biol. 2020. PMID: 32707065 Free PMC article.
-
Genetic control of sex differences in C. elegans neurobiology and behavior.Adv Genet. 2007;59:1-37. doi: 10.1016/S0065-2660(07)59001-2. Adv Genet. 2007. PMID: 17888793 Review.
Cited by
-
Shifts in the distribution of mass densities is a signature of caloric restriction in Caenorhabditis elegans.PLoS One. 2013 Jul 29;8(7):e69651. doi: 10.1371/journal.pone.0069651. Print 2013. PLoS One. 2013. PMID: 23922767 Free PMC article.
-
Expression of Genes Encoding the Enzymes for Glycogen and Trehalose Metabolism in L3 and L4 Larvae of Anisakis simplex.J Parasitol Res. 2015;2015:438145. doi: 10.1155/2015/438145. Epub 2015 Dec 8. J Parasitol Res. 2015. PMID: 26783451 Free PMC article.
-
High-glucose diets have sex-specific effects on aging in C. elegans: toxic to hermaphrodites but beneficial to males.Aging (Albany NY). 2015 Jun;7(6):383-8. doi: 10.18632/aging.100759. Aging (Albany NY). 2015. PMID: 26143626 Free PMC article.
-
Lipid and Carbohydrate Metabolism in Caenorhabditis elegans.Genetics. 2017 Oct;207(2):413-446. doi: 10.1534/genetics.117.300106. Genetics. 2017. PMID: 28978773 Free PMC article. Review.
-
The nutritional requirements of Caenorhabditis elegans.Genes Nutr. 2019 May 6;14:15. doi: 10.1186/s12263-019-0637-7. eCollection 2019. Genes Nutr. 2019. PMID: 31080524 Free PMC article. Review.
References
-
- Ward S, Carrel JS (1979) Fertilization and sperm competition in the nematode Caenorhabditis elegans. Dev Biol 73: 304–321. - PubMed
-
- Sulston JE, Albertson DG, Thomson JN (1980) The Caenorhabditis elegans male: postembryonic development of nongonadal structures. Dev Biol 78: 542–576. - PubMed
-
- Sulston JE, Horvitz HR (1977) Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol 56: 110–156. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

