Are patent medicine vendors effective agents in malaria control? Using lot quality assurance sampling to assess quality of practice in Jigawa, Nigeria
- PMID: 22984555
- PMCID: PMC3440361
- DOI: 10.1371/journal.pone.0044775
Are patent medicine vendors effective agents in malaria control? Using lot quality assurance sampling to assess quality of practice in Jigawa, Nigeria
Abstract
Background: Patent medicine vendors (PMV) provide antimalarial treatment and care throughout Sub-Saharan Africa, and can play an important role in the fight against malaria. Their close-to-client infrastructure could enable lifesaving artemisinin-based combination therapy (ACT) to reach patients in time. However, systematic assessments of drug sellers' performance quality are crucial if their role is to be managed within the health system. Lot quality assurance sampling (LQAS) could be an efficient method to monitor and evaluate PMV practice, but has so far never been used for this purpose.
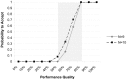
Methods: In support of the Nigeria Malaria Booster Program we assessed PMV practices in three Senatorial Districts (SDs) of Jigawa, Nigeria. A two-stage LQAS assessed whether at least 80% of PMV stores in SDs used national treatment guidelines. Acceptable sampling errors were set in consultation with government officials (alpha and beta <0.10). The hypergeometric formula determined sample sizes and cut-off values for SDs. A structured assessment tool identified high and low performing SDs for quality of care indicators.
Findings: Drug vendors performed poorly in all SDs of Jigawa for all indicators. For example, all SDs failed for stocking and selling first-line antimalarials. PMV sold no longer recommended antimalarials, such as Chloroquine, Sulfadoxine-Pyrimethamine and oral Artesunate monotherapy. Most PMV were ignorant of and lacked training about new treatment guidelines that had endorsed ACTs as first-line treatment for uncomplicated malaria.
Conclusion: There is urgent need to regularly monitor and improve the availability and quality of malaria treatment provided by medicine sellers in Nigeria; the irrational use of antimalarials in the ACT era revealed in this study bears a high risk of economic loss, death and development of drug resistance. LQAS has been shown to be a suitable method for monitoring malaria-related indicators among PMV, and should be applied in Nigeria and elsewhere to improve service delivery.
Conflict of interest statement
Figures
References
-
- Okeke TA, Okeibunor JC (2010) Rural-urban differences in health-seeking for the treatment of childhood malaria in south-east Nigeria. Health Policy 95: 62–68. - PubMed
-
- World Health Organization (2006) Partnerships for malaria control: engaging the formal and informal private sectors. Geneva.
-
- Oladepo O, Kabiru S, Adeoye BW, Oshiname F, Ofi B, et al. (2008) Malaria Treatment in Nigeria: The Role of Patent Medicine Vendors. Future Health Systems (FHS), Innovations for equity. Innovations and knowledge for future health systems for the poor, Policy Brief, March 2008 No (1) 1–4.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

