Immune complex signatures of patients with active and inactive SLE revealed by multiplex protein binding analysis on antigen microarrays
- PMID: 22984570
- PMCID: PMC3439431
- DOI: 10.1371/journal.pone.0044824
Immune complex signatures of patients with active and inactive SLE revealed by multiplex protein binding analysis on antigen microarrays
Abstract
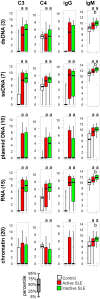
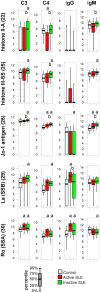
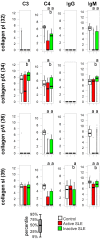
Systemic lupus erythematosus is characterized by dysfunctional clearance of apoptotic debris and the development of pathogenic autoantibodies. While the complement system is also involved in the disease no attempt has been made to generate a comprehensive view of immune complex formation from various autoantigens. We increased the complexity of autoantibody profiles by measuring the binding of two complement proteins, C3 and C4, in addition to two antibody classes, IgG and IgM, to a collection of autoantigens. These complement components covalently bind to those microarray features where antibodies and other serum components induce complement activation. Using this technology, we compared functional serum antibody profiles of control subjects (n = 31) and patients with lupus erythematosus (n = 61) in the active (n = 22) and inactive (n = 39) phase of the disease. Multivariate analysis was applied to identify contributions of binding data on 25 antigens to the discrimination of the study groups. Receiver operating characteristic analysis was used to portray the discriminative property of each measured parameter for each antigen in pairwise group comparisons. Complement C3 and C4 deposition increased on autoantibody targets in spite of the decreased serum complement concentrations, and decreased on other autoantigens, demonstrating the imbalance of complement function in patients with lupus erythematosus. Our observations confirmed previously known markers of disease and showed that C3 and C4 deposition data were at least as powerful as Ig binding data in separating the study groups.
Conflict of interest statement
Figures




References
-
- Hochberg MC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40: 1725. - PubMed
-
- Smeenk R, Brinkman K, van den Brink H, Termaat RM, Berden J, et al. (1990) Antibodies to DNA in patients with systemic lupus erythematosus. Their role in the diagnosis, the follow-up and the pathogenesis of the disease. Clin Rheumatol 9: 100–110. - PubMed
-
- Sturfelt G, Truedsson L (2005) Complement and its breakdown products in SLE. Rheumatology (Oxford) 44: 1227–1232. - PubMed
-
- Truedsson L, Bengtsson AA, Sturfelt G (2007) Complement deficiencies and systemic lupus erythematosus. Autoimmunity 40: 560–566. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

