Phosphoinositide 3-kinase C2β regulates RhoA and the actin cytoskeleton through an interaction with Dbl
- PMID: 22984590
- PMCID: PMC3440356
- DOI: 10.1371/journal.pone.0044945
Phosphoinositide 3-kinase C2β regulates RhoA and the actin cytoskeleton through an interaction with Dbl
Abstract
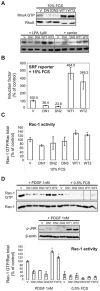
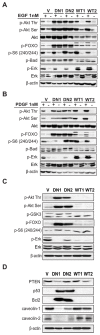
The regulation of cell morphology is a dynamic process under the control of multiple protein complexes acting in a coordinated manner. Phosphoinositide 3-kinases (PI3K) and their lipid products are widely involved in cytoskeletal regulation by interacting with proteins regulating RhoGTPases. Class II PI3K isoforms have been implicated in the regulation of the actin cytoskeleton, although their exact role and mechanism of action remain to be established. In this report, we have identified Dbl, a Rho family guanine nucleotide exchange factor (RhoGEF) as an interaction partner of PI3KC2β. Dbl was co-immunoprecipitated with PI3KC2β in NIH3T3 cells and cancer cell lines. Over-expression of Class II phosphoinositide 3-kinase PI3KC2β in NIH3T3 fibroblasts led to increased stress fibres formation and cell spreading. Accordingly, we found high basal RhoA activity and increased serum response factor (SRF) activation downstream of RhoA upon serum stimulation. In contrast, the dominant-negative form of PI3KC2β strongly reduced cell spreading and stress fibres formation, as well as SRF response. Platelet-derived growth factor (PDGF) stimulation of wild-type PI3KC2β over-expressing NIH3T3 cells strongly increased Rac and c-Jun N-terminal kinase (JNK) activation, but failed to show similar effect in the cells with the dominant-negative enzyme. Interestingly, epidermal growth factor (EGF) and PDGF stimulation led to increased extracellular signal-regulated kinase (Erk) and Akt pathway activation in cells with elevated wild-type PI3KC2β expression. Furthermore, increased expression of PI3KC2β protected NIH3T3 from detachment-dependent death (anoikis) in a RhoA-dependent manner. Taken together, these findings suggest that PI3KC2β modulates the cell morphology and survival through a specific interaction with Dbl and the activation of RhoA.
Conflict of interest statement
Figures





References
-
- Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, et al. (2001) Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol 17: 615–675. - PubMed
-
- Wymann MP, Bjorklof K, Calvez R, Finan P, Thomast M, et al. (2003) Phosphoinositide 3-kinase gamma: a key modulator in inflammation and allergy. Biochem Soc Trans 31: 275–280. - PubMed
-
- Sasaki T, Suzuki A, Sasaki J, Penninger JM (2002) Phosphoinositide 3-kinases in immunity: lessons from knockout mice. J Biochem 131: 495–501. - PubMed
-
- DiNitto JP, Cronin TC, Lambright DG (2003) Membrane recognition and targeting by lipid-binding domains. Sci STKE 2003: re16. - PubMed
-
- Engelman JA, Luo J, Cantley LC (2006) The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet 7: 606–619. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

