Astrocyte senescence as a component of Alzheimer's disease
- PMID: 22984612
- PMCID: PMC3440417
- DOI: 10.1371/journal.pone.0045069
Astrocyte senescence as a component of Alzheimer's disease
Abstract
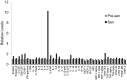
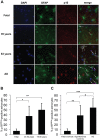
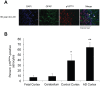
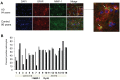
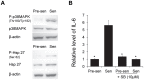
Aging is the main risk factor for Alzheimer's disease (AD); however, the aspects of the aging process that predispose the brain to the development of AD are largely unknown. Astrocytes perform a myriad of functions in the central nervous system to maintain homeostasis and support neuronal function. In vitro, human astrocytes are highly sensitive to oxidative stress and trigger a senescence program when faced with multiple types of stress. In order to determine whether senescent astrocytes appear in vivo, brain tissue from aged individuals and patients with AD was examined for the presence of senescent astrocytes using p16(INK4a) and matrix metalloproteinase-1 (MMP-1) expression as markers of senescence. Compared with fetal tissue samples (n = 4), a significant increase in p16(INK4a)-positive astrocytes was observed in subjects aged 35 to 50 years (n = 6; P = 0.02) and 78 to 90 years (n = 11; P<10(-6)). In addition, the frontal cortex of AD patients (n = 15) harbored a significantly greater burden of p16(INK4a)-positive astrocytes compared with non-AD adult control subjects of similar ages (n = 25; P = 0.02) and fetal controls (n = 4; P<10(-7)). Consistent with the senescent nature of the p16(INK4a)-positive astrocytes, increased metalloproteinase MMP-1 correlated with p16(INK4a). In vitro, beta-amyloid 1-42 (Aβ(1-42)) triggered senescence, driving the expression of p16(INK4a) and senescence-associated beta-galactosidase. In addition, we found that senescent astrocytes produce a number of inflammatory cytokines including interleukin-6 (IL-6), which seems to be regulated by p38MAPK. We propose that an accumulation of p16(INK4a)-positive senescent astrocytes may link increased age and increased risk for sporadic AD.
Conflict of interest statement
Figures

References
-
- Thies W, Bleiler L (2011) 2011 Alzheimer’s disease facts and figures. Alzheimers Dement 7: 208–244. - PubMed
-
- Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM (2006) Cellular senescence in aging primates. Science 311: 1257. - PubMed
-
- Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, et al. (2005) BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 436: 720–724. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

