A common clathrin-mediated machinery co-ordinates cell-cell adhesion and bacterial internalization
- PMID: 22984946
- PMCID: PMC3760411
- DOI: 10.1111/tra.12009
A common clathrin-mediated machinery co-ordinates cell-cell adhesion and bacterial internalization
Abstract
Invasive bacterial pathogens often target cellular proteins involved in adhesion as a first event during infection. For example, Listeria monocytogenes uses the bacterial protein InlA to interact with E-cadherin, hijack the host adherens junction (AJ) machinery and invade non-phagocytic cells by a clathrin-dependent mechanism. Here, we investigate a potential role for clathrin in cell-cell adhesion. We observed that the initial steps of AJ formation trigger the phosphorylation of clathrin, and its transient localization at forming cell-cell contacts. Furthermore, we show that clathrin serves as a hub for the recruitment of proteins that are necessary for the actin rearrangements that accompany the maturation of AJs. Using an InlA/E-cadherin chimera, we show that adherent cells expressing the chimera form AJs with cells expressing E-cadherin. We demonstrate that non-adherent cells expressing the InlA chimera, as bacteria, can be internalized by E-cadherin-expressing adherent cells. Together these results reveal that a common clathrin-mediated machinery may regulate internalization and cell adhesion and that the relative mobility of one of the interacting partners plays an important role in the commitment to either one of these processes.
© 2012 John Wiley & Sons A/S.
Figures

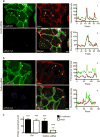

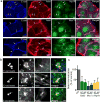

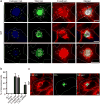

References
-
- Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–132. - PubMed
-
- Knodler LA, Celli J, Finlay BB. Pathogenic trickery: deception of host cell processes. Nat Rev Mol Cell Biol. 2001;2:578–588. - PubMed
-
- Pizarro-Cerdá J, Cossart P. Bacterial adhesion and entry into host cells. Cell. 2006;124:715–727. - PubMed
-
- Bonazzi M, Veiga E, Pizarro-Cerdá J, Cossart P. Successive post-translational modifications of E-cadherin are required for InlA-mediated internalization of Listeria monocytogenes. Cell Microbiol. 2008;10:2208–2222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

