Optimization of a small tropomyosin-related kinase B (TrkB) agonist 7,8-dihydroxyflavone active in mouse models of depression
- PMID: 22984948
- PMCID: PMC3491656
- DOI: 10.1021/jm301099x
Optimization of a small tropomyosin-related kinase B (TrkB) agonist 7,8-dihydroxyflavone active in mouse models of depression
Abstract
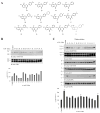
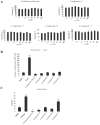
Structure-activity relationship study shows that the catechol group in 7,8-dihdyroxyflavone, a selective small TrkB receptor agonist, is critical for agonistic activity. To improve the poor pharmacokinetic profiles intrinsic to catechol-containing molecules and to elevate the agonistic effect of the lead compound, we initiated the lead optimization campaign by synthesizing various bioisosteric derivatives. Here we show that the optimized 2-methyl-8-(4'-(pyrrolidin-1-yl)phenyl)chromeno[7,8-d]imidazol-6(1H)-one derivative possesses enhanced TrkB stimulatory activity. Chronic oral administration of this compound significantly reduces the immobility in forced swim test and tail suspension test, two classical antidepressant behavioral animal models, which is accompanied by robust TrkB activation in hippocampus of mouse brain. Further, in vitro ADMET studies demonstrate that this compound possesses the improved features compared to the previous lead compound. Hence, this optimized compound may act as a promising lead candidate for in-depth drug development for treating various neurological disorders including depression.
Figures




Similar articles
-
Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation.Int J Neuropsychopharmacol. 2014 Oct 31;18(4):pyu077. doi: 10.1093/ijnp/pyu077. Int J Neuropsychopharmacol. 2014. PMID: 25628381 Free PMC article.
-
O-methylated metabolite of 7,8-dihydroxyflavone activates TrkB receptor and displays antidepressant activity.Pharmacology. 2013;91(3-4):185-200. doi: 10.1159/000346920. Epub 2013 Feb 21. Pharmacology. 2013. PMID: 23445871 Free PMC article.
-
A synthetic 7,8-dihydroxyflavone derivative promotes neurogenesis and exhibits potent antidepressant effect.J Med Chem. 2010 Dec 9;53(23):8274-86. doi: 10.1021/jm101206p. Epub 2010 Nov 12. J Med Chem. 2010. PMID: 21073191 Free PMC article.
-
7,8-Dihydroxyflavone reduces sleep during dark phase and suppresses orexin A but not orexin B in mice.J Psychiatr Res. 2015 Oct;69:110-9. doi: 10.1016/j.jpsychires.2015.08.002. Epub 2015 Aug 4. J Psychiatr Res. 2015. PMID: 26343602
-
Fisetin provides antidepressant effects by activating the tropomyosin receptor kinase B signal pathway in mice.J Neurochem. 2017 Dec;143(5):561-568. doi: 10.1111/jnc.14226. J Neurochem. 2017. PMID: 28945929
Cited by
-
Small-molecule TrkB receptor agonists improve motor function and extend survival in a mouse model of Huntington's disease.Hum Mol Genet. 2013 Jun 15;22(12):2462-70. doi: 10.1093/hmg/ddt098. Epub 2013 Feb 27. Hum Mol Genet. 2013. PMID: 23446639 Free PMC article.
-
Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation.Int J Neuropsychopharmacol. 2014 Oct 31;18(4):pyu077. doi: 10.1093/ijnp/pyu077. Int J Neuropsychopharmacol. 2014. PMID: 25628381 Free PMC article.
-
Radioligands for Tropomyosin Receptor Kinase (Trk) Positron Emission Tomography Imaging.Pharmaceuticals (Basel). 2019 Jan 3;12(1):7. doi: 10.3390/ph12010007. Pharmaceuticals (Basel). 2019. PMID: 30609832 Free PMC article. Review.
-
Protection of spiral ganglion neurons from degeneration using small-molecule TrkB receptor agonists.J Neurosci. 2013 Aug 7;33(32):13042-52. doi: 10.1523/JNEUROSCI.0854-13.2013. J Neurosci. 2013. PMID: 23926258 Free PMC article.
-
A Combined Computational and Experimental Approach to Studying Tropomyosin Kinase Receptor B Binders for Potential Treatment of Neurodegenerative Diseases.Molecules. 2024 Aug 23;29(17):3992. doi: 10.3390/molecules29173992. Molecules. 2024. PMID: 39274839 Free PMC article.
References
-
- Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, Agerman K, Haapasalo A, Nawa H, Aloyz R, Ernfors P, Castren E. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23:349–357. - PMC - PubMed
-
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

