Circulating hematopoietic stem cell count is a valuable predictor of prematurity complications in preterm newborns
- PMID: 22985188
- PMCID: PMC3573966
- DOI: 10.1186/1471-2431-12-148
Circulating hematopoietic stem cell count is a valuable predictor of prematurity complications in preterm newborns
Abstract
Background: The frequency of preterm labour has risen over the last few years. Hence, there is growing interest in the identification of markers that may facilitate prediction and prevention of premature birth complications. Here, we studied the association of the number of circulating stem cell populations with the incidence of complications typical of prematurity.
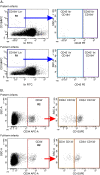
Methods: The study groups consisted of 90 preterm (23-36 weeks of gestational age) and 52 full-term (37-41 weeks) infants. Non-hematopoietic stem cells (non-HSCs; CD45-lin-CD184+), enriched in very small embryonic-like stem cells (VSELs), expressing pluripotent (Oct-4, Nanog), early neural (β-III-tubulin), and oligodendrocyte lineage (Olig-1) genes as well as hematopoietic stem cells (HSCs; CD45+lin-CD184+), and circulating stem/progenitor cells (CSPCs; CD133+CD34+; CD133-CD34+) in association with characteristics of prematurity and preterm morbidity were analyzed in cord blood (CB) and peripheral blood (PB) until the sixth week after delivery. Phenotype analysis was performed using flow cytometry methods. Clonogenic assays suitable for detection of human hematopoietic progenitor cells were also applied. The quantitative parameters were compared between groups by the Mann-Whitney test and between time points by the Friedman test. Fisher's exact test was used for qualitative variables.
Results: We found that the number of CB non-HSCs/VSELs is inversely associated with the birth weight of preterm infants. More notably, a high number of CB HSCs is strongly associated with a lower risk of prematurity complications including intraventricular hemorrhage, respiratory distress syndrome, infections, and anemia. The number of HSCs remains stable for the first six weeks of postnatal life. Besides, the number of CSPCs in CB is significantly higher in preterm infants than in full-term neonates (p < 0.0001) and extensively decreases in preterm babies during next six weeks after birth. Finally, the growth of burst-forming unit of erythrocytes (BFU-E) and colony-forming units of granulocyte-macrophage (CFU-GM) obtained from CB of premature neonates is higher than those obtained from CB of full-term infants and strongly correlates with the number of CB-derived CSPCs.
Conclusion: We conclude that CB HSCs are markedly associated with the development of premature birth complications. Thus, HSCs ought to be considered as the potential target for further research as they may be relevant for predicting and controlling the morbidity of premature infants. Moreover, the observed levels of non-HSCs/VSELs circulating in CB are inversely associated with the birth weight of preterm infants, suggesting non-HSCs/VSELs might be involved in the maturation of fetal organism.
Figures




References
-
- Paczkowska E, Kucia M, Koziarska D, Halasa M, Safranow K, Masiuk M, Karbicka A, Nowik M, Nowacki P, Ratajczak MZ, Machalinski B. Clinical evidence that very small embryonic-like (VSEL) stem cells are mobilized into peripheral blood in patients after stroke. Stroke. 2009;40:1237–1244. doi: 10.1161/STROKEAHA.108.535062. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

