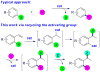
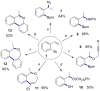
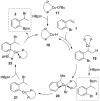
Copper-catalyzed recycling of halogen activating groups via 1,3-halogen migration
- PMID: 22985198
- PMCID: PMC4490833
- DOI: 10.1021/ja306446m
Copper-catalyzed recycling of halogen activating groups via 1,3-halogen migration
Abstract
A Cu(I)-catalyzed 1,3-halogen migration reaction effectively recycles an activating group by transferring bromine or iodine from a sp(2) to a benzylic carbon with concomitant borylation of the Ar-X bond. The resulting benzyl halide can be reacted in the same vessel under a variety of conditions to form an additional carbon-heteroatom bond. Cross-over experiments using an isotopically enriched bromide source support intramolecular transfer of Br. The reaction is postulated to proceed via a Markovnikov hydrocupration of the o-halostyrene, oxidative addition of the resulting Cu(I) complex into the Ar-X bond, reductive elimination of the new sp(3) C-X bond, and final borylation of an Ar-Cu(I) species to turn over the catalytic cycle.
Figures
References
-
- Negishi Ei, de Meijere A., editors. Handbook of Organopalladium Chemistry for Organic Synthesis. Wiley; New York: 2002.
- de Meijere A, Diederich F, editors. Metal-Catalyzed Cross-Coupling Reactions. 2nd. Wiley-VCH; Weinheim: 2004.
-
-
Selected reviews of C-H functionalization: Mkhalid IA, Barnard JH, Marder TB, Murphy JM, Hartwig JF. Chem Rev. 2010;110:890.Lyons TW, Sanford MS. Chem Rev. 2010;110:1147.Engle KM, Mei TS, Wasa M, Yu JQ. Acc Chem Res. 2012;45:788.Neufeldt SR, Sanford MS. Acc Chem Res. 2012;45:936.Song G, Wang F, Li X. Chem Soc Rev. 2012;41:3651.Ackermann L. Chem Rev. 2011;111:1315.Davies HML, Morton D. Chem Soc Rev. 2011;40:1857.Wendlandt AE, Suess AM, Stahl SS. Angew Chem Int Ed. 2011;50:11062.
-
-
- Bedford RB, Coles SJ, Hursthouse MB, Limmert ME. Angew Chem Int Ed. 2003;42:112. - PubMed
- Ihara H, Suginome M. J Am Chem Soc. 2009;131:7502. - PubMed
- Robbins DW, Boebel TA, Hartwig JF. J Am Chem Soc. 2010;132:4068. - PubMed
- Dai HX, Stepan AF, Plummer MF, Zhang YH, Yu JQ. J Am Chem Soc. 2011;133:7222. - PubMed
- Huang C, Chattopadhyay B, Grevorgyan V. J Am Chem Soc. 2011;133:12406. - PMC - PubMed
- Gulevich AV, Melkonyan FS, Sarkar D, Gevorgyan V. J Am Chem Soc. 2012;134:5528. - PMC - PubMed
-
-
Recent examples of activating group recycling: Newman SG, Lautens M. J Am Chem Soc. 2011;133:1778.Hooper JF, Chaplin AB, González-Rodríguez C, Thompson AL, Weller AS, Willis MC. J Am Chem Soc. 2012;134:2906.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials