Comparative in vitro and in vivo efficacies of human simulated doses of ceftazidime and ceftazidime-avibactam against Pseudomonas aeruginosa
- PMID: 22985878
- PMCID: PMC3497209
- DOI: 10.1128/AAC.00851-12
Comparative in vitro and in vivo efficacies of human simulated doses of ceftazidime and ceftazidime-avibactam against Pseudomonas aeruginosa
Abstract
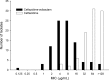
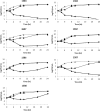
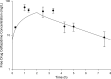
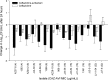
The combination of ceftazidime and avibactam possesses potent activity against resistant Gram-negative pathogens, including Pseudomonas aeruginosa. We compared the efficacies of human simulated doses of ceftazidime and ceftazidime-avibactam using a hollow-fiber system and neutropenic and immunocompetent murine thigh infection models. Twenty-seven clinical P. aeruginosa isolates with ceftazidime MICs of 8 to 128 mg/liter and ceftazidime-avibactam MICs of 4 to 32 mg/liter were utilized in neutropenic mouse studies; 15 of the isolates were also evaluated in immunocompetent mice. Six isolates were studied in both the hollow-fiber system and the neutropenic mouse. In both systems, the free drug concentration-time profile seen in humans given 2 g of ceftazidime every 8 h (2-h infusion), with or without avibactam at 500 mg every 8 h (2-h infusion), was evaluated. In vivo activity was pharmacodynamically predictable based on the MIC. Ceftazidime decreased bacterial densities by ≥0.5 log unit for 10/27 isolates, while ceftazidime-avibactam did so for 22/27 isolates. In immunocompetent animals, enhancements in activity were seen for both drugs, with ceftazidime achieving reductions of ≥0.3 log unit for 10/15 isolates, whereas ceftazidime-avibactam did so against all 15 isolates. In vitro, ceftazidime resulted in regrowth by 24 h against all isolates, while ceftazidime-avibactam achieved stasis or better against 4/7 isolates. Mutants with elevated ceftazidime-avibactam MICs appeared after 24 h from 3/7 isolates studied in vitro; however, no resistant mutants were detected in vivo. Against this highly ceftazidime-nonsusceptible population of P. aeruginosa, treatment with human simulated doses of ceftazidime-avibactam resulted in pharmacodynamically predictable activity, particularly in vivo, against isolates with MICs of ≤16 mg/liter, and this represents a potential new option to combat these difficult-to-treat pathogens.
Figures




References
-
- Blaser J, Stone BB, Groner MC, Zinner SH. 1987. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob. Agents Chemother. 31:1054–1060 - PMC - PubMed
-
- Blaser J, Stone BB, Zinner SH. 1985. Two compartment kinetic model with multiple artificial capillary units. J. Antimicrob. Chemother. 15(Suppl A):131–137 - PubMed
-
- Clinical Laboratory Standard Institute 2011. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 8th ed CLSI publication M07-A8. Clinical Laboratory Standard Institute, Wayne, PA
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

