Effect of continuous venovenous hemofiltration dose on achievement of adequate vancomycin trough concentrations
- PMID: 22985887
- PMCID: PMC3497205
- DOI: 10.1128/AAC.00459-12
Effect of continuous venovenous hemofiltration dose on achievement of adequate vancomycin trough concentrations
Abstract
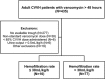
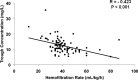
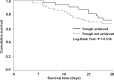
The vancomycin dose necessary for the achievement of target serum trough concentrations during continuous venovenous hemofiltration (CVVH) remains to be elucidated. This was a retrospective cohort study of critically ill adults at a tertiary medical center on concurrent CVVH and vancomycin between 2006 and 2010 with a steady-state vancomycin trough concentration. The 87 included patients were grouped according to low (≤30 ml/kg/h; n = 10) or high (>30 ml/kg/h; n = 77) CVVH hemofiltration rate (HFR) for analysis. Vancomycin goal trough achievement occurred in only 32 (37%) patients. The primary endpoint of trough attainment significantly differed between HFR subgroups: 90% versus 30% in low- and high-HFR individuals, respectively (P < 0.001). Patients with subtherapeutic trough concentrations had a median (interquartile range) HFR of 40 ml/kg/h (range, 37 to 47 ml/kg/h) compared to 36 ml/kg/h (range, 30 to 39 ml/kg/h) in those who achieved the trough goal. Irrespective of goal trough, an inverse correlation existed between HFR and serum vancomycin concentration (r = -0.423; P < 0.001). In the subgroup of 14 methicillin-resistant Staphylococcus aureus (MRSA) patients, trough achievement was similar to the aggregate cohort (36%). Mortality at 28 days was unrelated to trough achievement in both the overall sample (P = 0.516) and in culture-positive MRSA patients (P = 0.396). Critically ill patients undergoing CVVH therapy may experience clinically significant reductions in goal vancomycin troughs. The results of the present study justify prospective evaluations in this population to determine the optimal vancomycin dosing strategy for attainment of goal trough concentrations.
Figures
Similar articles
-
Evaluation of dosing strategies and trough concentrations of vancomycin in patients undergoing continuous venovenous hemofiltration.Pharmacotherapy. 2021 Jul;41(7):554-561. doi: 10.1002/phar.2535. Epub 2021 Jun 18. Pharmacotherapy. 2021. PMID: 33963536
-
Vancomycin clearance in high-volume venovenous hemofiltration.Ann Pharmacother. 2013 Mar;47(3):e14. doi: 10.1345/aph.1Q488. Epub 2013 Feb 12. Ann Pharmacother. 2013. PMID: 23404801
-
Effect of Extracorporeal Membrane Oxygenation on the New Vancomycin Dosing Regimen in Critically Ill Patients Receiving Continuous Venovenous Hemofiltration.Ther Drug Monit. 2018 Jun;40(3):310-314. doi: 10.1097/FTD.0000000000000495. Ther Drug Monit. 2018. PMID: 29746432
-
[Therapeutic monitoring of vancomycin in routine clinical practice].Vnitr Lek. 2014 Oct;60(10):846-51. Vnitr Lek. 2014. PMID: 25382007 Review. Czech.
-
The Whole Price of Vancomycin: Toxicities, Troughs, and Time.Drugs. 2017 Jul;77(11):1143-1154. doi: 10.1007/s40265-017-0764-7. Drugs. 2017. PMID: 28573434 Free PMC article. Review.
Cited by
-
Vancomycin therapeutic drug monitoring in patients on continuous renal replacement therapy: a retrospective study.J Int Med Res. 2022 Sep;50(9):3000605221126871. doi: 10.1177/03000605221126871. J Int Med Res. 2022. PMID: 36177821 Free PMC article.
-
Case report: therapeutic monitoring of vancomycin in an acute liver failure patient with anuria under high-flow continuous hemodiafiltration.J Pharm Health Care Sci. 2023 May 1;9(1):15. doi: 10.1186/s40780-023-00283-0. J Pharm Health Care Sci. 2023. PMID: 37122008 Free PMC article.
-
Adequacy of cefepime concentrations in the early phase of critical illness: A case for precision pharmacotherapy.Pharmacotherapy. 2023 Nov;43(11):1112-1120. doi: 10.1002/phar.2766. Epub 2023 Feb 2. Pharmacotherapy. 2023. PMID: 36648390 Free PMC article.
-
Factors affecting serum concentration of vancomycin in critically ill oliguric pediatric patients receiving continuous venovenous hemodiafiltration.PLoS One. 2018 Jun 21;13(6):e0199158. doi: 10.1371/journal.pone.0199158. eCollection 2018. PLoS One. 2018. PMID: 29927988 Free PMC article.
-
Continuous infusion versus intermittent infusion of vancomycin in critically ill patients undergoing continuous venovenous hemofiltration: a prospective interventional study.BMC Infect Dis. 2022 Aug 2;22(1):667. doi: 10.1186/s12879-022-07618-6. BMC Infect Dis. 2022. PMID: 35918657 Free PMC article. Clinical Trial.
References
-
- Ali T, et al. 2007. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J. Am. Soc. Nephrol. 18:1292–1298 - PubMed
-
- Angus DC, et al. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29:1303–1310 - PubMed
-
- Bagshaw SM, George C, Bellomo R. 2008. A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol. Dial. Transplant. 23:1569–1574 - PubMed
-
- Bellomo R, et al. 2009. Intensity of continuous renal-replacement therapy in critically ill patients. N. Engl. J. Med. 361:1627–1638 - PubMed
-
- Boereboom FT, Ververs FF, Blankestijn PJ, Savelkoul TJ, van Dijk A. 1999. Vancomycin clearance during continuous venovenous haemofiltration in critically ill patients. Intensive Care Med. 25:1100–1104 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical