Insights towards sulfonamide drug specificity in α-carbonic anhydrases
- PMID: 22985956
- PMCID: PMC3593968
- DOI: 10.1016/j.bmc.2012.08.019
Insights towards sulfonamide drug specificity in α-carbonic anhydrases
Abstract
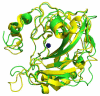
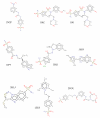
Carbonic anhydrases (CAs, EC 4.2.1.1) are a group of metalloenzymes that play important roles in carbon metabolism, pH regulation, CO2 fixation in plants, ion transport etc., and are found in all eukaryotic and many microbial organisms. This family of enzymes catalyzes the interconversion of CO2 and HCO3(-). There are at least 16 different CA isoforms in the alpha structural class (α-CAs) that have been isolated in higher vertebrates, with CA isoform II (CA II) being ubiquitously abundant in all human cell types. CA inhibition has been exploited clinically for decades for various classes of diuretics and anti-glaucoma treatment. The characterization of the overexpression of CA isoform IX (CA IX) in certain tumors has raised interest in CA IX as a diagnostic marker and drug target for aggressive cancers and therefore the development of CA IX specific inhibitors. An important goal in the field of CA is to identify, rationalize, and design potential compounds that will preferentially inhibit CA IX over all other isoforms of CA. The variations in the active sites between isoforms of CA are subtle and this causes non-specific CA inhibition which leads to various side effects. In the case of CA IX inhibition, CA II along with other isoforms of CA provide off-target binding sites which is undesirable for cancer treatment. The focus of this article is on CA IX inhibition and two different structural approaches to CA isoform specific drug designing: tail approach and fragment addition approach.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures





References
-
- Messerchmidt A, Bode W, Cygler M. Handbook of Metalloproteins. John Wiley and Sons Ltd.; 2004.
-
- Scozzafava A, Mastrolorenzo A, Supuran CT. Expert Opinion on Therapeutic Patents. 2006;16:1627–1664. - PubMed
-
- Supuran CT. Nat. Rev. Drug Discov. 2008;7(2):168–81. - PubMed
- Supuran CT, Scozzafava A, Conway J. Carbonic anhydrase: its inhibitors and activators. CRC Press; 2004.
-
- Pastorekova S, Parkkila S, Pastorek J, Supuran CT. J Enzyme Inhib Med Chem. 2004;19:199–229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

