The syndromes of reduced sensitivity to thyroid hormone
- PMID: 22986150
- PMCID: PMC3528849
- DOI: 10.1016/j.bbagen.2012.08.005
The syndromes of reduced sensitivity to thyroid hormone
Abstract
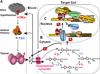
Background: Six known steps are required for the circulating thyroid hormone (TH) to exert its action on target tissues. For three of these steps, human mutations and distinct phenotypes have been identified.
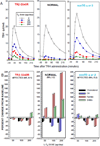
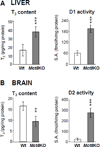
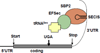
Scope of review: The clinical, laboratory, genetic and molecular characteristics of these three defects of TH action are the subject of this review. The first defect, recognized 45years ago, produces resistance to TH and carries the acronym, RTH. In the majority of cases it is caused by TH receptor β gene mutations. It has been found in over 3000 individuals belonging to approximately 1000 families. Two relatively novel syndromes presenting reduced sensitivity to TH involve membrane transport and metabolism of TH. One of them, caused by mutations in the TH cell-membrane transporter MCT8, produces severe psychomotor defects. It has been identified in more than 170 males from 90 families. A defect of the intracellular metabolism of TH in 10 individuals from 8 families is caused by mutations in the SECISBP2 gene required for the synthesis of selenoproteins, including TH deiodinases.
Major conclusions: Defects at different steps along the pathway leading to TH action at cellular level can manifest as reduced sensitivity to TH.
General significance: Knowledge of the molecular mechanisms involved in TH action allows the recognition of the phenotypes caused by defects of TH action. Once previously known defects have been ruled out, new molecular defects could be sought, thus opening the avenue for novel insights in thyroid physiology. This article is part of a Special Issue entitled Thyroid hormone signaling.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures





References
-
- Refetoff S, DeWind LT, DeGroot LJ. Familial syndrome combining deaf-mutism, stippled epiphyses, goiter, and abnormally high PBI: possible target organ refractoriness to thyroid hormone. J Clin Endocrinol Metab. 1967;27:279–294. - PubMed
-
- Refetoff S, DeGroot LJ, Benard B, DeWind LT. Studies of a sibship with apparent hereditary resistance to the intracellular action of thyroid hormone. Metabolism. 1972;21:723–756. - PubMed
-
- Weinberger C, Thompson CC, Ong ES, Lebo R, Gruol DJ, Evans RM. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986;324:641–646. - PubMed
-
- Sap J, Munoz A, Schmitt J, Stunnenberg H, Vennström B. Repression of transcription mediated at thyroid hormone response element by the v-erbA oncogene product. Nature. 1989;340:242–244. - PubMed
-
- Sakurai A, Takeda K, Ain K, Ceccarelli P, Nakai A, Seino S, Bell GI, Refetoff S, DeGroot LJ. Generalized resistance to thyroid hormone associated with a mutation in the ligand-binding domain of the human thyroid hormone receptor b. Procedings of the National Academy of Sciences (USA) 1989;86:8977–8981. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

