Promoter-proximal pausing of RNA polymerase II: emerging roles in metazoans
- PMID: 22986266
- PMCID: PMC3552498
- DOI: 10.1038/nrg3293
Promoter-proximal pausing of RNA polymerase II: emerging roles in metazoans
Abstract
Recent years have witnessed a sea change in our understanding of transcription regulation: whereas traditional models focused solely on the events that brought RNA polymerase II (Pol II) to a gene promoter to initiate RNA synthesis, emerging evidence points to the pausing of Pol II during early elongation as a widespread regulatory mechanism in higher eukaryotes. Current data indicate that pausing is particularly enriched at genes in signal-responsive pathways. Here the evidence for pausing of Pol II from recent high-throughput studies will be discussed, as well as the potential interconnected functions of promoter-proximally paused Pol II.
Figures
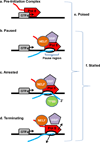
Pre-Initiation Complex: an entry form of Pol II in complex with general transcription factors, where the polymerase is bound to the promoter DNA but has not yet initiated RNA synthesis.
Paused: an early elongation complex that has transiently halted RNA synthesis. Paused polymerase is fully competent to resume elongation, remaining stably engaged and associated with the nascent RNA. The 3’-end of the RNA may have ‘frayed’ slightly from the Pol II active site in a manner that would slow further RNA synthesis, but the RNA is properly aligned with the active site. Two protein complexes, DSIF and NELF reduce the rate of elongation and facilitate the establishment of the stably paused state.
Arrested: a stably engaged elongation complex wherein the polymerase has backtracked along the DNA template, such that the RNA 3’-end is displaced from the active site. Re-start of an arrested complex usually requires the transcript cleavage factor TFIIS, which induces Pol II to cleave the nascent RNA at the active site, creating a new 3’-end that is properly aligned with the Pol II active site and releasing a short (2–9 nt) 3’ RNA.
Terminating: an unstable elongation complex that is in the process of dissociating from the DNA template and releasing the nascent RNA. The released Pol II could have the potential to rapidly re-initiate transcription and “re-cycle” at the promoter.
Poised: very generic term that simply indicates that Pol II is located near the TSS, but does not specify anything about its transcriptional status. Can include any of the above complexes (a–d).
Stalled: term indicating that Pol II is engaged in transcription, but that makes no assumptions about its ability to resume synthesis. This term includes paused, arrested and terminating complexes (b–d, above).
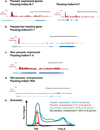
Two paused, but active genes with differing pausing indices
A paused but inactive gene
A Non-paused gene that is expressed
A Non-paused, unexpressed gene. We note that a pausing index cannot reliably be calculated for genes that lack significant Pol II promoter signal.
Shown are profiles of Pol II signal that exemplify the four major groups of genes. We note that the “paused, unexpressed” group is significantly under-represented in vivo, suggesting that most paused genes display some basal RNA synthesis. Approximate percentages of genes that fall into each category are given, , .
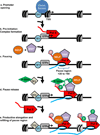
Promoter opening often involves the binding of a sequence specific transcription factor (shown here as TF1, light blue circle) that brings in chromatin remodelers (blue oval) to remove nucleosomes from around the TSS and render the promoter accessible for recruitment of the transcription machinery.
Pre-Initiation Complex (PIC) formation involves the recruitment of a set of general transcription factors (GTFs, grey oval) and Pol II which is also facilitated by the binding of specific transcription factors (also shown as TF1 for simplicity). This step precedes the initiation of RNA synthesis.
Pol II pausing occurs shortly after transcription initiation and involves the association of pausing factors DSIF and NELF. The paused Pol II is phosphorylated on its CTD (shown in pink). The region wherein pausing takes place is shown by a bracket.
Pause release is triggered by the recruitment of the P-TEFb kinase (green diamond), either directly or indirectly by a transcription factor (shown here as TF2, tan diamond). P-TEFb kinase phosphorylates the DSIF/NELF complex to release paused Pol II and also targets the CTD (shown in green). Phosphorylation of DSIF/NELF dissociates NELF from the elongation complex and transforms DSIF into a positive elongation factor that associates with Pol II throughout the gene.
In the presence of both TF1 and TF2, escape of the paused Pol II into productive elongation is followed rapidly by entry of another Pol II into the pause site, allowing for efficient RNA production. When the gene is activated, some nucleosome disruption is likely, as depicted by the lighter colouring of the downstream nucleosome.
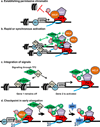
Establishing permissive chromatin: in the absence of paused Pol II, nucleosomes occlude the promoter region and inhibit gene expression, as shown in the left hand panel. Once, these nucleosomes are remodelled, paused Pol II helps to maintain the nucleosome-deprived structure by blocking nucleosome assembly over promoter sequences (depicted at right). Pausing thus would keep the promoter region accessible for activator and transcription factor binding.
Rapid or synchronous gene activation: at a gene with paused Pol II, gene activation could proceed simply through recruitment of P-TEFb, thereby triggering the rapid release of paused Pol II into productive elongation. If a number of genes harbouring paused Pol II were all activated by the same signal and associated transcription factor (shown as TF2), then these genes could activated simultaneously and in a rapid fashion.
Integrating multiple regulatory signals: Pausing represents a separate step in the transcription cycle for factors to act, and allows for combinatorial control between transcription factors that recruit the transcription machinery (TF1) and those that trigger pause release (TF2), where both would be necessary for gene activation. In this example, signalling through TF2 such that it binds DNA and recruits P-TEFb would not lead to activation of a gene that was not paused (i.e. lacks TF1), but would stimulate synthesis from Gene 2 that was loaded with paused polymerase.
Checkpoint in early elongation: on the left, arrows depict interactions between the Capping Enzyme Complex (CEC) and DSIF/NELF as well as the Ser5-P modification of the CTD of Pol II, which is thought to stimulate capping activity. The hat represents the 5’ RNA cap. Center, P-TEFb dependent phosphorylation events release paused Pol II, and create a platform for binding of RNA processing factors (RPF) on the Ser2-P CTD of Pol II, as shown at right.
References
-
- Lis J. Promoter-associated pausing in promoter architecture and postinitiation transcriptional regulation. Cold Spring Harb Symp Quant Biol. 1998;63:347–356. - PubMed
-
-
Core LJ, Waterfall JJ, Lis JT. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science. 2008;322:1845–1848.. Global run-on sequencing (GRO-seq) maps the position, amount, and orientation of transcriptionally engaged RNA polymerases genome-wide, and shows peaks of promoter-proximal polymerase residing on ~30% of human genes.
-
-
-
Gilchrist DA, et al. Pausing of RNA polymerase II disrupts DNA-specified nucleosome organization to enable precise gene regulation. Cell. 2010;143:540–551.. Global analyses of Pol II pausing and nucleosome occupancy reveal that Pol II and nucleosomes compete for promoter occupancy to co-ordinately regulate gene expression.
-
-
-
Lee C, et al. NELF and GAGA factor are linked to promoter-proximal pausing at many genes in Drosophila. Mol Cell Biol. 2008;28:3290–3300.. Comprehensive analysis of promoter-associated Pol II in Drosophila using ChIP-chip and permanganate demonstrates that NELF-mediated pausing of Pol II is common in Drosophila.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

