The cellular and molecular basis for malaria parasite invasion of the human red blood cell
- PMID: 22986493
- PMCID: PMC3444787
- DOI: 10.1083/jcb.201206112
The cellular and molecular basis for malaria parasite invasion of the human red blood cell
Abstract
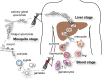
Malaria is a major disease of humans caused by protozoan parasites from the genus Plasmodium. It has a complex life cycle; however, asexual parasite infection within the blood stream is responsible for all disease pathology. This stage is initiated when merozoites, the free invasive blood-stage form, invade circulating erythrocytes. Although invasion is rapid, it is the only time of the life cycle when the parasite is directly exposed to the host immune system. Significant effort has, therefore, focused on identifying the proteins involved and understanding the underlying mechanisms behind merozoite invasion into the protected niche inside the human erythrocyte.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

