Traumatic brain injury in Latin America: lifespan analysis randomized control trial protocol*
- PMID: 22986600
- PMCID: PMC3549327
- DOI: 10.1227/NEU.0b013e31827276b7
Traumatic brain injury in Latin America: lifespan analysis randomized control trial protocol*
Abstract
Background: Although in the developed world the intracranial pressure (ICP) monitor is considered the standard of care for patients with severe traumatic brain injury (TBI), its usefulness to direct treatment decisions has never been tested rigorously.
Objective: The primary focus was to conduct a high-quality, randomized, controlled trial to determine whether ICP monitoring used to direct TBI treatment improves patient outcomes. By providing education, equipment, and structure, the project will enhance the research capacity of the collaborating investigators and will foster the collaborations established during earlier studies.
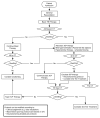
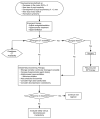
Methods: Study centers were selected that routinely treated ICP based on clinical examination and computed tomography imaging using internal protocols. We randomized patients to either an ICP monitor group or an imaging and clinical examination group. Treatment decisions for the ICP monitor group are guided by ICP monitoring based on established guidelines. Treatment decisions for the imaging and clinical examination group are made using a single protocol derived from those previously being used at those centers.
Expected outcomes: There are 2 study hypotheses: (1) patients with severe TBI whose acute care treatment is managed using ICP monitors will have improved outcomes and 2) incorporating ICP monitoring in the care of patients with severe TBI will minimize complications and decrease length of intensive care unit stay.
Discussion: This clinical trial tests the effectiveness of a management protocol based on technology considered pivotal to brain trauma treatment in the developed world: the ICP monitor. A randomized, controlled trial of ICP monitoring has never been performed-a critical gap in the evidence base that supports the role of ICP monitoring in TBI care. As such, the results of this randomized, controlled trial will have global implications regardless of the level of development of the trauma system.
Figures
References
-
- Bratton SL, Chesnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(Suppl 1):S1–106. - PubMed
-
- O’Brien PC. Procedures for comparing samples with multiple endpoints. Biometrics. 1984;40:1079–1087. - PubMed
-
- Klove H, editor. Grooved Pegboard. Lafayette Instruments; 1963.
-
- Rappaport M, Hall KM, Hopkins K, Belleza T, Cope DN. Disability rating scale for severe head trauma: coma to community. Arch Phys Med Rehabil Mar. 1982;63(3):118–123. - PubMed
-
- Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. Journal of Neurotrauma. 1998;15(8):573–585. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous