Purinergic receptor antagonist A438079 protects against acetaminophen-induced liver injury by inhibiting p450 isoenzymes, not by inflammasome activation
- PMID: 22986947
- PMCID: PMC3537131
- DOI: 10.1093/toxsci/kfs283
Purinergic receptor antagonist A438079 protects against acetaminophen-induced liver injury by inhibiting p450 isoenzymes, not by inflammasome activation
Abstract
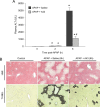
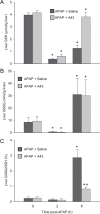
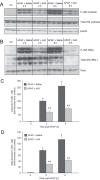
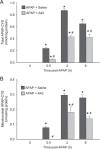
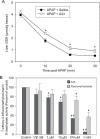
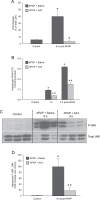
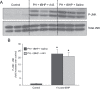
Acetaminophen (APAP) overdose is the most frequent cause of acute liver failure in the western world. Controversy exists regarding the hypothesis that the hepatocyte injury is amplified by a sterile inflammatory response, rather than being the result of intracellular mechanisms alone. A recent study suggested that the purinergic receptor antagonist A438079 protects against APAP-induced liver injury by preventing the activation of the Nalp3 inflammasome in Kupffer cells and thereby preventing inflammatory injury. To test the hypothesis that A438079 actually affects the intracellular signaling events in hepatocytes, C57Bl/6 mice were treated with APAP (300 mg/kg) and A438079 (80 mg/kg) or saline and GSH depletion, protein adduct formation, c-jun-N-terminal kinase (JNK) activation, oxidant stress, and liver cell necrosis were determined between 0 and 6 h after APAP administration. APAP caused rapid GSH depletion, extensive protein adduct formation in liver homogenates and in mitochondria, JNK phosphorylation and mitochondrial translocation of phospho-JNK within 2 h, oxidant stress, and extensive centrilobular necrosis at 6 h. A438079 significantly attenuated GSH depletion, which resulted in a 50% reduction of total liver and mitochondrial protein adducts and substantial reduction of JNK activation, mitochondrial P-JNK translocation, oxidant stress, and liver injury. The same results were obtained using primary mouse hepatocytes. A438079 did not directly affect JNK activation induced by tert-butyl hydroperoxide and GSH depletion. However, A438079 dose-dependently inhibited hepatic P450 enzyme activity. Thus, the protective effect of A438079 against APAP hepatotoxicity in vivo can be explained by its effect on metabolic activation and cell death pathways in hepatocytes without involvement of the Nalp3 inflammasome.
Figures
References
-
- Bajt M. L., Knight T. R., Lemasters J. J., Jaeschke H. (2004). Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: Protection by N-acetyl cysteine. Toxicol. Sci. 80, 343–349. - PubMed
-
- Bajt M. L., Lawson J. A., Vonderfecht S. L., Gujral J. S., Jaeschke H. (2000). Protection against Fas receptor-mediated apoptosis in hepatocytes and nonparenchymal cells by a caspase-8 inhibitor in vivo: Evidence for a postmitochondrial processing of caspase-8. Toxicol. Sci. 58, 109–117. - PubMed
-
- Buters J. T., Schiller C. D., Chou R. C. (1993). A highly sensitive tool for the assay of cytochrome P450 enzyme activity in rat, dog and man. Direct fluorescence monitoring of the deethylation of 7-ethoxy-4-trifluoromethylcoumarin. Biochem. Pharmacol. 46, 1577–1584. - PubMed
-
- Cohen S. D., Pumford N. R., Khairallah E. A., Boekelheide K., Pohl L. R., Amouzadeh H. R., Hinson J. A. (1997). Selective protein covalent binding and target organ toxicity. Toxicol. Appl. Pharmacol. 143, 1–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

