Characterization of human Spartan/C1orf124, an ubiquitin-PCNA interacting regulator of DNA damage tolerance
- PMID: 22987070
- PMCID: PMC3510514
- DOI: 10.1093/nar/gks850
Characterization of human Spartan/C1orf124, an ubiquitin-PCNA interacting regulator of DNA damage tolerance
Abstract
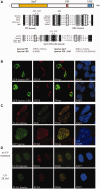
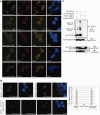
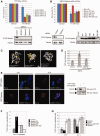
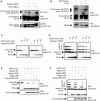
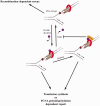
Unrepaired DNA damage may arrest ongoing replication forks, potentially resulting in fork collapse, increased mutagenesis and genomic instability. Replication through DNA lesions depends on mono- and polyubiquitylation of proliferating cell nuclear antigen (PCNA), which enable translesion synthesis (TLS) and template switching, respectively. A proper replication fork rescue is ensured by the dynamic ubiquitylation and deubiquitylation of PCNA; however, as yet, little is known about its regulation. Here, we show that human Spartan/C1orf124 protein provides a higher cellular level of ubiquitylated-PCNA by which it regulates the choice of DNA damage tolerance pathways. We find that Spartan is recruited to sites of replication stress, a process that depends on its PCNA- and ubiquitin-interacting domains and the RAD18 PCNA ubiquitin ligase. Preferential association of Spartan with ubiquitin-modified PCNA protects against PCNA deubiquitylation by ubiquitin-specific protease 1 and facilitates the access of a TLS polymerase to the replication fork. In concert, depletion of Spartan leads to increased sensitivity to DNA damaging agents and causes elevated levels of sister chromatid exchanges. We propose that Spartan promotes genomic stability by regulating the choice of rescue of stalled replication fork, whose mechanism includes its interaction with ubiquitin-conjugated PCNA and protection against PCNA deubiquitylation.
Figures

References
-
- Prakash S, Johnson RE, Prakash L. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu. Rev. Biochem. 2005;74:317–353. - PubMed
-
- Bailly V, Lamb J, Sung P, Prakash S, Prakash L. Specific complex-formation between yeast Rad6 and Rad18 proteins—a potential mechanism for targeting Rad6 ubiquitin-conjugating activity to DNA-damage sites. Genes Dev. 1994;8:811–820. - PubMed
-
- Prakash S, Prakash L. Translesion DNA synthesis in eukaryotes: a one- or two-polymerase affair. Genes Dev. 2002;16:1872–1883. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

