Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia
- PMID: 22987078
- PMCID: PMC3518729
- DOI: 10.1200/JCO.2012.43.4738
Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia
Abstract
Purpose: To evaluate the prognostic significance of the international European LeukemiaNet (ELN) guidelines for reporting genetic alterations in acute myeloid leukemia (AML).
Patients and methods: We analyzed 1,550 adults with primary AML, treated on Cancer and Leukemia Group B first-line trials, who had pretreatment cytogenetics and, for cytogenetically normal patients, mutational status of NPM1, CEBPA, and FLT3 available. We compared complete remission (CR) rates, disease-free survival (DFS), and overall survival (OS) among patients classified into the four ELN genetic groups (favorable, intermediate-I, intermediate-II, adverse) separately for 818 younger (age < 60 years) and 732 older (age ≥ 60 years) patients.
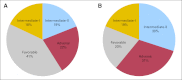
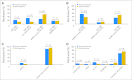
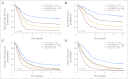
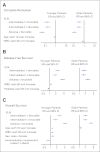
Results: The percentages of younger versus older patients in the favorable (41% v 20%; P < .001), intermediate-II (19% v 30%; P < .001), and adverse (22% v 31%; P < .001) genetic groups differed. The favorable group had the best and the adverse group the worst CR rates, DFS, and OS in both age groups. Both intermediate groups had significantly worse outcomes than the favorable but better than the adverse group. Intermediate-I and intermediate-II groups in older patients had similar outcomes, whereas the intermediate-II group in younger patients had better OS but not better CR rates or DFS than the intermediate-I group. The prognostic significance of ELN classification was confirmed by multivariable analyses. For each ELN group, older patients had worse outcomes than younger patients.
Conclusion: The ELN classification clearly separates the genetic groups by outcome, supporting its use for risk stratification in clinical trials. Because they have different proportions of genetic alterations and outcomes, younger and older patients should be reported separately when using the ELN classification.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Haematological cancer: European LeukemiaNet AML genetic classification works.Nat Rev Clin Oncol. 2012 Nov;9(11):607. doi: 10.1038/nrclinonc.2012.180. Epub 2012 Oct 2. Nat Rev Clin Oncol. 2012. PMID: 23026876 No abstract available.
-
Molecular cytogenetics and multiplex reverse-transcriptase polymerase chain reaction for risk stratification in acute myeloid leukemia.J Clin Oncol. 2013 Jun 20;31(18):2360-1. doi: 10.1200/JCO.2013.48.8189. Epub 2013 May 6. J Clin Oncol. 2013. PMID: 23650414 No abstract available.
-
Reply to K. Orendi et al.J Clin Oncol. 2013 Jun 20;31(18):2361-2. doi: 10.1200/JCO.2013.49.2504. J Clin Oncol. 2013. PMID: 23930275 No abstract available.
References
-
- Mrózek K, Heerema NA, Bloomfield CD. Cytogenetics in acute leukemia. Blood Rev. 2004;18:115–136. - PubMed
-
- Smith ML, Hills RK, Grimwade D. Independent prognostic variables in acute myeloid leukaemia. Blood Rev. 2011;25:39–51. - PubMed
-
- Schlenk RF, Döhner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–1918. - PubMed
-
- Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: Analysis of 1,612 patients entered into the MRC AML 10 trial. Blood. 1998;92:2322–2333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 CA033601/CA/NCI NIH HHS/United States
- U24 CA114725/CA/NCI NIH HHS/United States
- U10 CA101140/CA/NCI NIH HHS/United States
- P30 CA016058/CA/NCI NIH HHS/United States
- CA77658/CA/NCI NIH HHS/United States
- CA31946/CA/NCI NIH HHS/United States
- CA140158/CA/NCI NIH HHS/United States
- CA33601/CA/NCI NIH HHS/United States
- P50 CA140158/CA/NCI NIH HHS/United States
- CA16058/CA/NCI NIH HHS/United States
- CA101140/CA/NCI NIH HHS/United States
- U10 CA077658/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- R21 CA129657/CA/NCI NIH HHS/United States
- CA129657/CA/NCI NIH HHS/United States
- CA114725/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

