Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer
- PMID: 22987084
- PMCID: PMC3478574
- DOI: 10.1200/JCO.2011.40.0010
Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer
Abstract
Purpose: Cardiac dysfunction (CD) is a recognized risk associated with the addition of trastuzumab to adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer, especially when the treatment regimen includes anthracyclines. Given the demonstrated efficacy of trastuzumab, ongoing assessment of cardiac safety and identification of risk factors for CD are important for optimal patient care.
Patients and methods: In National Surgical Adjuvant Breast and Bowel Project B-31, a phase III adjuvant trial, 1,830 patients who met eligibility criteria for initiation of trastuzumab were evaluated for CD. Recovery from CD was also assessed. A statistical model was developed to estimate the risk of severe congestive heart failure (CHF). Baseline patient characteristics associated with anthracycline-related decline in cardiac function were also identified.
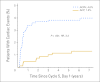
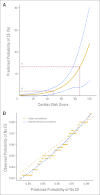
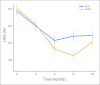
Results: At 7-year follow-up, 37 (4.0%) of 944 patients who received trastuzumab experienced a cardiac event (CE) versus 10 (1.3%) of 743 patients in the control arm. One cardiac-related death has occurred in each arm of the protocol. A Cardiac Risk Score, calculated using patient age and baseline left ventricular ejection fraction (LVEF) by multiple-gated acquisition scan, statistically correlates with the risk of a CE. After stopping trastuzumab, the majority of patients who experienced CD recovered LVEF in the normal range, although some decline from baseline often persists. Only two CEs occurred more than 2 years after initiation of trastuzumab.
Conclusion: The late development of CHF after the addition of trastuzumab to paclitaxel after doxorubicin/ cyclophosphamide chemotherapy is uncommon. The risk versus benefit of trastuzumab as given in this regimen remains strongly in favor of trastuzumab.
Trial registration: ClinicalTrials.gov NCT00004067.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


Comment in
-
Long term follow-up of national surgical adjuvant breast and bowel project trial B-31: how well can we predict cardiac toxicity with trastuzumab?J Clin Oncol. 2012 Nov 1;30(31):3769-72. doi: 10.1200/JCO.2012.44.9611. Epub 2012 Sep 17. J Clin Oncol. 2012. PMID: 22987088 No abstract available.
-
Questionable validity of cardiac risk score on the basis of the NSABP B-31 model.J Clin Oncol. 2013 Jun 10;31(17):2224. doi: 10.1200/JCO.2012.47.2696. Epub 2013 May 6. J Clin Oncol. 2013. PMID: 23650420 No abstract available.
-
Reply to V. Pitini et al.J Clin Oncol. 2013 Jun 10;31(17):2225. doi: 10.1200/JCO.2012.48.3701. J Clin Oncol. 2013. PMID: 23901419 No abstract available.
References
-
- Piccart-Gebhart MJ, Proctor M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. - PubMed
-
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. - PubMed
-
- Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2–overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005;23:7811–7819. - PubMed
-
- van Royen N, Jaffe CC, Krumholz HM, et al. Comparison and reproducibility of visual echocardiographic and quantitative radionuclide left ventricular ejection fractions. Am J Cardiol. 1996;77:843–850. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

