Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice
- PMID: 22987396
- PMCID: PMC3566276
- DOI: 10.1002/hep.26081
Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice
Abstract
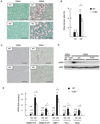
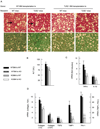
Innate immune signaling associated with Toll-like receptors (TLRs) is a key pathway involved in the progression of nonalcoholic steatohepatitis (NASH). Here we show that both TLR2 and palmitic acid are required for activation of the inflammasome, interleukin (IL)-1α, and IL-1β, resulting in the progression of NASH. Wild-type (WT) and TLR2(-/-) mice were fed a choline-deficient amino acid-defined (CDAA) diet for 22 weeks to induce NASH. Bone marrow-transplanted TLR2 chimeric mice were generated after the recipient mice were lethally irradiated. Kupffer cells and hepatic stellate cells (HSCs) were isolated from WT mice and stimulated with TLR2 ligand and/or palmitic acid. WT mice on the CDAA diet developed profound steatohepatitis and liver fibrosis. In contrast, TLR2(-/-) mice had suppressed progression of NASH. Although both Kupffer cells and HSCs respond to TLR2 ligand, TLR2 bone marrow chimeric mice demonstrated that Kupffer cells were relatively more important than HSCs in TLR2-mediated progression of NASH. In vitro, palmitic acid alone did not increase TLR2 signaling-target genes, including cytokines and inflammasome components in Kupffer cells and HSCs. The TLR2 ligand increased Nod-like receptor protein 3, an inflammasome component, in Kupffer cells but not in HSCs. In the presence of TLR2 ligand, palmitic acid did induce caspase-1 activation and release of IL-1α and IL-1β in Kupffer cells; however, these effects were not observed in HSCs. In vivo, WT mice on the CDAA diet showed increased caspase-1 activation in the liver and elevated serum levels of IL-1α and IL-1β levels, which were suppressed in TLR2(-/-) mice.
Conclusion: TLR2 and palmitic acid cooperatively activate the inflammasome in Kupffer cells and/or macrophages in the development of NASH.
Copyright © 2012 American Association for the Study of Liver Diseases.
Conflict of interest statement
No conflict of interest
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
