Layer V perirhinal cortical ensemble activity during object exploration: a comparison between young and aged rats
- PMID: 22987683
- PMCID: PMC3523702
- DOI: 10.1002/hipo.22066
Layer V perirhinal cortical ensemble activity during object exploration: a comparison between young and aged rats
Abstract
Object recognition memory requires the perirhinal cortex (PRC) and this cognitive function declines during normal aging. Recent electrophysiological recordings from young rats have shown that neurons in Layer V of the PRC are activated by three-dimensional objects. Thus, it is possible that age-related object recognition deficits result from alterations in PRC neuron activity in older animals. To examine this, the present study used cellular compartment analysis of temporal activity by fluorescence in situ hybridization (catFISH) with confocal microscopy to monitor cellular distributions of activity-induced Arc RNA in layer V of the PRC. Activity was monitored during two distinct epochs of object exploration. In one group of rats (6 young/6 aged) animals were placed in a familiar testing arena and allowed to explore five different three-dimensional objects for two 5-min sessions separated by a 20-min rest (AA). The second group of animals (6 young/6 aged) also explored the same objects for two 5-min sessions, but the environment was changed between the first and the second epoch (AB). Behavioral data showed that both age groups spent less time exploring objects during the second epoch, even when the environment changed, indicating successful recognition. Although the proportion of active neurons between epochs did not change in the AA group, in the AB group more neurons were active during epoch 2 of object exploration. This recruitment of neurons into the active neural ensemble could serve to signal that familiar stimuli are being encountered in a new context. When numbers of Arc positive neurons were compared between age groups, the old rats had significantly lower proportions of Arc-positive PRC neurons in both the AA and AB behavioral conditions. These data support the hypothesis that age-associated functional alterations in the PRC contribute to declines in stimulus recognition over the lifespan.
Copyright © 2012 Wiley Periodicals, Inc.
Figures



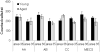






References
-
- Aggleton JP, Kyd RJ, Bilkey DK. When is the perirhinal cortex necessary for the performance of spatial memory tasks? Neurosci Biobehav Rev. 2004;28(6):611–24. - PubMed
-
- Barnes CA, Rao G, McNaughton BL. Functional integrity of NMDA-dependent LTP induction mechanisms across the lifespan of F-344 rats. Learn Mem. 1996;3(2-3):124–37. - PubMed
-
- Barnes CA, Suster MS, Shen J, McNaughton BL. Multistability of cognitive maps in the hippocampus of old rats. Nature. 1997;388(6639):272–5. - PubMed
-
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2(1):51–61. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

