Review of MR elastography applications and recent developments
- PMID: 22987755
- PMCID: PMC3462370
- DOI: 10.1002/jmri.23597
Review of MR elastography applications and recent developments
Abstract
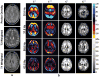
The technique of MR elastography (MRE) has emerged as a useful modality for quantitatively imaging the mechanical properties of soft tissues in vivo. Recently, MRE has been introduced as a clinical tool for evaluating chronic liver disease, but many other potential applications are being explored. These applications include measuring tissue changes associated with diseases of the liver, breast, brain, heart, and skeletal muscle including both focal lesions (e.g., hepatic, breast, and brain tumors) and diffuse diseases (e.g., fibrosis and multiple sclerosis). The purpose of this review article is to summarize some of the recent developments of MRE and to highlight some emerging applications.
Copyright © 2012 Wiley Periodicals, Inc.
Figures







References
-
- American Cancer Society. Cancer facts & figures. Atlanta, GA: American Cancer Society, Inc; 2008. p. 70.
-
- Fatemi M, Manduca A, Greenleaf JF. Imaging elastic properties of biological tissues by low-frequency harmonic vibration. Proc IEEE. 2003;91:1503–1519.
-
- Greenleaf JF, Fatemi M, Insana M. Selected methods for imaging elastic properties of biological tissues. Annu Rev Biomed Eng. 2003;5:57–78. - PubMed
-
- Parker KJ, Doyley MM, Rubens DJ. Imaging the elastic properties of tissue: the 20 year perspective. Phys Med Biol. 2011;56:R1–R29. - PubMed
-
- Chenevert TL, Skovoroda AR, O’Donnell M, Emelianov SY. Elasticity reconstructive imaging by means of stimulated echo MRI. Magnet Reson Med. 1998;39:482–490. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

