High-throughput lensfree 3D tracking of human sperms reveals rare statistics of helical trajectories
- PMID: 22988076
- PMCID: PMC3479566
- DOI: 10.1073/pnas.1212506109
High-throughput lensfree 3D tracking of human sperms reveals rare statistics of helical trajectories
Abstract
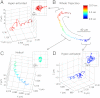
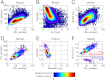
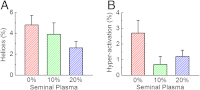
Dynamic tracking of human sperms across a large volume is a challenging task. To provide a high-throughput solution to this important need, here we describe a lensfree on-chip imaging technique that can track the three-dimensional (3D) trajectories of > 1,500 individual human sperms within an observation volume of approximately 8-17 mm(3). This computational imaging platform relies on holographic lensfree shadows of sperms that are simultaneously acquired at two different wavelengths, emanating from two partially-coherent sources that are placed at 45° with respect to each other. This multiangle and multicolor illumination scheme permits us to dynamically track the 3D motion of human sperms across a field-of-view of > 17 mm(2) and depth-of-field of approximately 0.5-1 mm with submicron positioning accuracy. The large statistics provided by this lensfree imaging platform revealed that only approximately 4-5% of the motile human sperms swim along well-defined helices and that this percentage can be significantly suppressed under seminal plasma. Furthermore, among these observed helical human sperms, a significant majority (approximately 90%) preferred right-handed helices over left-handed ones, with a helix radius of approximately 0.5-3 μm, a helical rotation speed of approximately 3-20 rotations/s and a linear speed of approximately 20-100 μm/s. This high-throughput 3D imaging platform could in general be quite valuable for observing the statistical swimming patterns of various other microorganisms, leading to new insights in their 3D motion and the underlying biophysics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
Words of wisdom: re: high-throughput lensfree 3D tracking of human sperms reveals rare statistics of helical trajectories.Eur Urol. 2013 Apr;63(4):768-9. doi: 10.1016/j.eururo.2012.12.054. Eur Urol. 2013. PMID: 23438393 No abstract available.
References
-
- Serres C, Feneux D, Jouannet P, David G. Influence of the flagellar wave development and propagation on the human sperm movement in seminal plasma. Gamete Res. 1984;9:183–195.
-
- Ishijima S, Oshio S, Mohri H. Flagellar movement of human spermatozoa. Gamete Res. 1986;13:185–197.
-
- Mortimer ST, Swan MA. Variable kinematics of capacitating human spermatozoa. Hum Reprod. 1995;10:3178–3182. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

