Synthesis of site-specific antibody-drug conjugates using unnatural amino acids
- PMID: 22988081
- PMCID: PMC3479532
- DOI: 10.1073/pnas.1211023109
Synthesis of site-specific antibody-drug conjugates using unnatural amino acids
Abstract
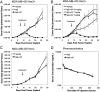
Antibody-drug conjugates (ADCs) allow selective targeting of cytotoxic drugs to cancer cells presenting tumor-associated surface markers, thereby minimizing systemic toxicity. Traditionally, the drug is conjugated nonselectively to cysteine or lysine residues in the antibody. However, these strategies often lead to heterogeneous products, which make optimization of the biological, physical, and pharmacological properties of an ADC challenging. Here we demonstrate the use of genetically encoded unnatural amino acids with orthogonal chemical reactivity to synthesize homogeneous ADCs with precise control of conjugation site and stoichiometry. p-Acetylphenylalanine was site-specifically incorporated into an anti-Her2 antibody Fab fragment and full-length IgG in Escherichia coli and mammalian cells, respectively. The mutant protein was selectively and efficiently conjugated to an auristatin derivative through a stable oxime linkage. The resulting conjugates demonstrated excellent pharmacokinetics, potent in vitro cytotoxic activity against Her2(+) cancer cells, and complete tumor regression in rodent xenograft treatment models. The synthesis and characterization of homogeneous ADCs with medicinal chemistry-like control over macromolecular structure should facilitate the optimization of ADCs for a host of therapeutic uses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Wu AM, Senter PD. Arming antibodies: Prospects and challenges for immunoconjugates. Nat Biotechnol. 2005;23:1137–1146. - PubMed
-
- Polakis P. Arming antibodies for cancer therapy. Curr Opin Pharmacol. 2005;5:382–387. - PubMed
-
- Lambert JM. Drug-conjugated monoclonal antibodies for the treatment of cancer. Curr Opin Pharmacol. 2005;5:543–549. - PubMed
-
- Vater CA, Goldmacher VS. Antibody-cytotoxic compound conjugates for oncology. Macromolecular Anticancer Therapeutics. 2010 Part 4:331–369.
-
- Kovtun YV, Goldmacher VS. Cell killing by antibody-drug conjugates. Cancer Lett. 2007;255:232–240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

