C-terminal region of the UV-B photoreceptor UVR8 initiates signaling through interaction with the COP1 protein
- PMID: 22988111
- PMCID: PMC3479605
- DOI: 10.1073/pnas.1210898109
C-terminal region of the UV-B photoreceptor UVR8 initiates signaling through interaction with the COP1 protein
Abstract
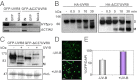
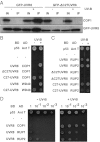
UV-B light initiates photomorphogenic responses in plants. Arabidopsis UV RESISTANCE LOCUS8 (UVR8) specifically mediates these responses by functioning as a UV-B photoreceptor. UV-B exposure converts UVR8 from a dimer to a monomer, stimulates the rapid accumulation of UVR8 in the nucleus, where it binds to chromatin, and induces interaction of UVR8 with CONSTITUTIVELY PHOTOMORPHOGENIC1 (COP1), which functions with UVR8 to control photomorphogenic UV-B responses. Although the crystal structure of UVR8 reveals the basis of photoreception, it does not show how UVR8 initiates signaling through interaction with COP1. Here we report that a region of 27 amino acids from the C terminus of UVR8 (C27) mediates the interaction with COP1. The C27 region is necessary for UVR8 function in the regulation of gene expression and hypocotyl growth suppression in Arabidopsis. However, UVR8 lacking C27 still undergoes UV-B-induced monomerization in both yeast and plant protein extracts, accumulates in the nucleus in response to UV-B, and interacts with chromatin at the UVR8-regulated ELONGATED HYPOCOTYL5 (HY5) gene. The UV-B-dependent interaction of UVR8 and COP1 is reproduced in yeast cells and we show that C27 is both necessary and sufficient for the interaction of UVR8 with the WD40 domain of COP1. Furthermore, we show that C27 interacts in yeast with the REPRESSOR OF UV-B PHOTOMORPHOGENESIS proteins, RUP1 and RUP2, which are negative regulators of UVR8 function. Hence the C27 region has a key role in UVR8 function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ulm R, Nagy F. Signalling and gene regulation in response to ultraviolet light. Curr Opin Plant Biol. 2005;8:477–482. - PubMed
-
- Jenkins GI. Signal transduction in responses to UV-B radiation. Annu Rev Plant Biol. 2009;60:407–431. - PubMed
-
- Heijde M, Ulm R. UV-B photoreceptor-mediated signalling in plants. Trends Plant Sci. 2012;17:230–237. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

