Association of TMEM16A chloride channel overexpression with airway goblet cell metaplasia
- PMID: 22988141
- PMCID: PMC3530122
- DOI: 10.1113/jphysiol.2012.240838
Association of TMEM16A chloride channel overexpression with airway goblet cell metaplasia
Abstract
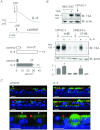
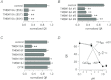
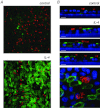
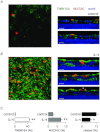
The TMEM16A protein has a potential role as a Ca(2+)-activated Cl(-) channel (CaCC) in airway epithelia where it may be important in the homeostasis of the airway surface fluid. We investigated the function and expression of TMEM16A in primary human bronchial epithelial cells and in a bronchial cell line (CFBE41o-). Under resting conditions, TMEM16A protein expression was relatively low. However, TMEM16A silencing with short-interfering RNAs caused a marked inhibition of CaCC activity, thus demonstrating that a low TMEM16A expression is sufficient to support Ca(2+)-dependent Cl(-) transport. Following treatment for 24-72 h with interleukin-4 (IL-4), a cytokine that induces mucous cell metaplasia, TMEM16A protein expression was strongly increased in approximately 50% of primary bronchial epithelial cells, with a specific localization in the apical membrane. IL-4 treatment also increased the percentage of cells expressing MUC5AC, a marker of goblet cells. Interestingly, MUC5AC was detected specifically in cells expressing TMEM16A. In particular, MUC5AC was found in 15 and 60% of TMEM16A-positive cells when epithelia were treated with IL-4 for 24 or 72 h, respectively. In contrast, ciliated cells showed expression of the cystic fibrosis transmembrane conductance regulator Cl(-) channel but not of TMEM16A. Our results indicate that TMEM16A protein is responsible for CaCC activity in airway epithelial cells, particularly in cells treated with IL-4, and that TMEM16A upregulation by IL-4 appears as an early event of goblet cell differentiation. These findings suggest that TMEM16A expression is particularly required under conditions of mucus hypersecretion to ensure adequate secretion of electrolytes and water.
Figures


Comment in
-
Time for TMEM?J Physiol. 2012 Dec 1;590(23):5931-2. doi: 10.1113/jphysiol.2012.245563. J Physiol. 2012. PMID: 23204100 Free PMC article. No abstract available.
References
-
- Billig GM, Pál B, Fidzinski P, Jentsch TJ. Ca2+-activated Cl− currents are dispensable for olfaction. Nat Neurosci. 2011;14:763–769. - PubMed
-
- Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004;23:146–158. - PubMed
-
- Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. - PubMed
-
- Cui L, Aleksandrov L, Chang XB, Hou YX, He L, Hegedus T, Gentzsch M, Aleksandrov A, Balch WE, Riordan JR. Domain interdependence in the biosynthetic assembly of CFTR. J Mol Biol. 2007;365:981–994. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

