Use of population based background rates of disease to assess vaccine safety in childhood and mass immunisation in Denmark: nationwide population based cohort study
- PMID: 22988304
- PMCID: PMC3444137
- DOI: 10.1136/bmj.e5823
Use of population based background rates of disease to assess vaccine safety in childhood and mass immunisation in Denmark: nationwide population based cohort study
Abstract
Objectives: To predict the number of selected outcomes temporally associated but not caused by vaccination, to aid causality assessment of adverse events arising after mass immunisation in a paediatric population.
Design: Nationwide population based cohort study.
Setting: Denmark.
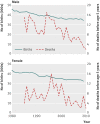
Participants: All liveborn infants delivered after 1 January 1980. Study population was followed from date of birth until hospital admission for selected outcome diagnoses, death, first emigration, age 18 years, or 31 December 2009. The study population was subject to vaccines used in standard childhood immunisation in Denmark, with 82-93% vaccine coverage.
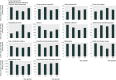
Main outcome measures: Incidence of acute infectious and post-infectious polyneuritis (Guillain-Barré syndrome), acute transverse myelitis, optic polyneuritis, facial nerve palsy, anaphylactic shock, seizure, multiple sclerosis, autoimmune thrombocytopenia, type 1 diabetes mellitus, juvenile and rheumatoid arthritis, narcolepsy, and death of unknown cause stratified by sex, age, and season. We predicted the number of events for a hypothetical vaccine cohort of 1,000,000 people for follow-up periods of up to 182 days.
Results: The study included 2,300,227 liveborn infants, yielding 37,262,404 person years of follow-up; median follow-up was 16.8 person years. Incidence of outcome diagnoses spanned from 0.32 per 100,000 patient years for autoimmune thrombocytopenia to 189.82 per 100,000 patient years for seizure. Seasonal differences were most pronounced for anaphylactic shock, seizure, and multiple sclerosis. Even for rare outcomes, numerous events were predicted in the hypothetical vaccine cohort. We predicted that 20 cases of type 1 diabetes mellitus, 19 of juvenile or rheumatoid arthritis, eight of facial nerve palsy, and five of multiple sclerosis per 1,000,000 children would occur within 42 days after vaccination.
Conclusions: Incorporating exact background rates of disease based on age, sex, and seasonal distribution could strengthen vaccine safety assessment, and provides an evidence based focus for discussing the incremental risk of newly introduced vaccines.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
Figures
Similar articles
-
Neurological and autoimmune disorders after vaccination against pandemic influenza A (H1N1) with a monovalent adjuvanted vaccine: population based cohort study in Stockholm, Sweden.BMJ. 2011 Oct 12;343:d5956. doi: 10.1136/bmj.d5956. BMJ. 2011. PMID: 21994316 Free PMC article.
-
Importance of background rates of disease in assessment of vaccine safety during mass immunisation with pandemic H1N1 influenza vaccines.Lancet. 2009 Dec 19;374(9707):2115-2122. doi: 10.1016/S0140-6736(09)61877-8. Epub 2009 Oct 31. Lancet. 2009. PMID: 19880172 Free PMC article.
-
Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study.BMJ. 2021 May 5;373:n1114. doi: 10.1136/bmj.n1114. BMJ. 2021. PMID: 33952445 Free PMC article.
-
Mortality and morbidity in patients with osteogenesis imperfecta in Denmark.Dan Med J. 2018 Apr;65(4):B5454. Dan Med J. 2018. PMID: 29619932 Review.
-
Postlicensure epidemiology of childhood vaccination: the Danish experience.Expert Rev Vaccines. 2006 Oct;5(5):641-9. doi: 10.1586/14760584.5.5.641. Expert Rev Vaccines. 2006. PMID: 17181438 Review.
Cited by
-
General Practitioner Attendance in Proximity to HPV Vaccination: A Nationwide, Register-Based, Matched Case-Control Study.Clin Epidemiol. 2020 Sep 8;12:929-939. doi: 10.2147/CLEP.S253429. eCollection 2020. Clin Epidemiol. 2020. PMID: 32982458 Free PMC article.
-
Autoimmune, neurological, and venous thromboembolic adverse events after immunisation of adolescent girls with quadrivalent human papillomavirus vaccine in Denmark and Sweden: cohort study.BMJ. 2013 Oct 9;347:f5906. doi: 10.1136/bmj.f5906. BMJ. 2013. PMID: 24108159 Free PMC article.
-
Multifactorial Modulation of Food-Induced Anaphylaxis.Front Immunol. 2017 May 16;8:552. doi: 10.3389/fimmu.2017.00552. eCollection 2017. Front Immunol. 2017. PMID: 28559894 Free PMC article. Review.
-
Safety of Streptococcus pyogenes Vaccines: Anticipating and Overcoming Challenges for Clinical Trials and Post-Marketing Monitoring.Clin Infect Dis. 2023 Sep 18;77(6):917-924. doi: 10.1093/cid/ciad311. Clin Infect Dis. 2023. PMID: 37232372 Free PMC article.
-
Coverage of the 2011 Q fever vaccination campaign in the Netherlands, using retrospective population-based prevalence estimation of cardiovascular risk-conditions for chronic Q fever.PLoS One. 2015 Apr 24;10(4):e0123570. doi: 10.1371/journal.pone.0123570. eCollection 2015. PLoS One. 2015. PMID: 25909712 Free PMC article.
References
-
- Wijnans L, de Bie S, Dieleman J, Bonhoeffer J, Sturkenboom M. Safety of pandemic H1N1 vaccines in children and adolescents. Vaccine 2011;29:7559-71. - PubMed
-
- Destefano F, Tokars J. H1N1 vaccine safety monitoring: beyond background rates. Lancet 2010;375:1146-7. - PubMed
-
- Lieu TA, Kulldorff M, Davis RL, Lewis EM, Weintraub E, Yih K, et al. Real-time vaccine safety surveillance for the early detection of adverse events. Med Care 2007;45(10 suppl 2):S89-95. - PubMed
-
- Salmon DA, Akhtar A, Mergler MJ, Vannice KS, Izurieta H, Ball R, et al. Immunization-safety monitoring systems for the 2009 H1N1 monovalent influenza vaccination program. Pediatrics 2011;127(suppl 1):S78-86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases