A genome-wide screen to identify transcription factors expressed in pelvic Ganglia of the lower urinary tract
- PMID: 22988430
- PMCID: PMC3439845
- DOI: 10.3389/fnins.2012.00130
A genome-wide screen to identify transcription factors expressed in pelvic Ganglia of the lower urinary tract
Abstract
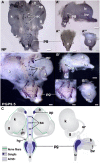
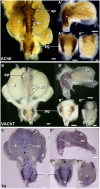
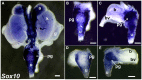
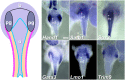
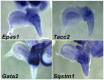
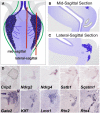
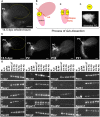
Relative positions of neurons within mature murine pelvic ganglia based on expression of neurotransmitters have been described. However the spatial organization of developing innervation in the murine urogenital tract (UGT) and the gene networks that regulate specification and maturation of neurons within the pelvic ganglia of the lower urinary tract (LUT) are unknown. We used whole-mount immunohistochemistry and histochemical stains to localize neural elements in 15.5 days post coitus (dpc) fetal mice. To identify potential regulatory factors expressed in pelvic ganglia, we surveyed expression patterns for known or probable transcription factors (TF) annotated in the mouse genome by screening a whole-mount in situ hybridization library of fetal UGTs. Of the 155 genes detected in pelvic ganglia, 88 encode TFs based on the presence of predicted DNA-binding domains. Neural crest (NC)-derived progenitors within the LUT were labeled by Sox10, a well-known regulator of NC development. Genes identified were categorized based on patterns of restricted expression in pelvic ganglia, pelvic ganglia and urethral epithelium, or pelvic ganglia and urethral mesenchyme. Gene expression patterns and the distribution of Sox10+, Phox2b+, Hu+, and PGP9.5+ cells within developing ganglia suggest previously unrecognized regional segregation of Sox10+ progenitors and differentiating neurons in early development of pelvic ganglia. Reverse transcription-PCR of pelvic ganglia RNA from fetal and post-natal stages demonstrated that multiple TFs maintain post-natal expression, although Pax3 is extinguished before weaning. Our analysis identifies multiple potential regulatory genes including TFs that may participate in segregation of discrete lineages within pelvic ganglia. The genes identified here are attractive candidate disease genes that may now be further investigated for their roles in malformation syndromes or in LUT dysfunction.
Keywords: Genitourinary Development Molecular Anatomy Project; autonomic nervous system; genitourinary; in situ hybridization; lower urinary tract; mouse; pelvic ganglia; transcription factor.
Figures


References
-
- Arvidsson U., Riedl M., Elde R., Meister B. (1997). Vesicular acetylcholine transporter (VAChT) protein: a novel and unique marker for cholinergic neurons in the central and peripheral nervous systems. J. Comp. Neurol. 378, 454–467 10.1002/(SICI)1096-9861(19970224)378:4<454::AID-CNE2>3.0.CO;2-1 - DOI - PubMed
-
- Bamforth S. D., Bragança J., Eloranta J. J., Murdoch J. N., Marques F. I., Kranc K. R., Farza H., Henderson D. J., Hurst H. C., Bhattacharya S. (2001). Cardiac malformations, adrenal agenesis, neural crest defects and exencephaly in mice lacking Cited2, a new Tfap2 co-activator. Nat. Genet. 29, 469–474 10.1038/ng768 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

