Human blood RNA stabilization in samples collected and transported for a large biobank
- PMID: 22988904
- PMCID: PMC3503553
- DOI: 10.1186/1756-0500-5-510
Human blood RNA stabilization in samples collected and transported for a large biobank
Abstract
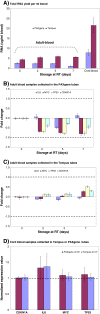
Background: The Norwegian Mother and Child Cohort Study (MoBa) is a nation-wide population-based pregnancy cohort initiated in 1999, comprising more than 108.000 pregnancies recruited between 1999 and 2008. In this study we evaluated the feasibility of integrating RNA analyses into existing MoBa protocols. We compared two different blood RNA collection tube systems - the PAXgene™ Blood RNA system and the Tempus™ Blood RNA system - and assessed the effects of suboptimal blood volumes in collection tubes and of transportation of blood samples by standard mail. Endpoints to characterize the samples were RNA quality and yield, and the RNA transcript stability of selected genes.
Findings: High-quality RNA could be extracted from blood samples stabilized with both PAXgene and Tempus tubes. The RNA yields obtained from the blood samples collected in Tempus tubes were consistently higher than from PAXgene tubes. Higher RNA yields were obtained from cord blood (3 - 4 times) compared to adult blood with both types of tubes. Transportation of samples by standard mail had moderate effects on RNA quality and RNA transcript stability; the overall RNA quality of the transported samples was high. Some unexplained changes in gene expression were noted, which seemed to correlate with suboptimal blood volumes collected in the tubes. Temperature variations during transportation may also be of some importance.
Conclusions: Our results strongly suggest that special collection tubes are necessary for RNA stabilization and they should be used for establishing new biobanks. We also show that the 50,000 samples collected in the MoBa biobank provide RNA of high quality and in sufficient amounts to allow gene expression analyses for studying the association of disease with altered patterns of gene expression.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

